Illustration by Enan Liang
Mitchell Gorby came into this world around 3 p.m. on August 9, 2019, at Balboa Naval Hospital in San Diego. The baby seemed healthy, and his parents, Tiffany and Rylan, were thrilled. But a few hours later a nurse noticed that Mitchell seemed lethargic and never cried, and monitors indicated that his body was not getting enough oxygen. Mitchell was rushed to the neonatal intensive care unit at nearby Rady Children’s Hospital, where tests revealed that oxygen wasn’t bonding to the molecule that carries it through the blood, hemoglobin, and his red blood cells were dying off. He wasn’t nursing, so the hospital put in a feeding tube. Mitchell’s doctor ordered CT and brain scans and tested for infectious diseases—but she could not figure out what was wrong with him. As a last resort, she suggested sequencing Mitchell’s genome.
The results from Stephen Kingsmore’s laboratory at the Rady Children’s Institute for Genomic Medicine came back within about 48 hours. Mitchell had a rare genetic mutation known as hemoglobin Toms River, which prevents oxygen from bonding to the proteins in fetal red blood cells. The mutation—named after the New Jersey hometown of the first patient identified with the problem in 2011—affects only fetal hemoglobin; babies start making healthy adult hemoglobin within a few months. Doctors just had to keep Mitchell alive until that happened. Rady neonatologist Jeanne Carroll says that “having his whole genome allowed us to know the starting point” for treatment. She and Mitchell’s team of physicians prescribed a series of blood transfusions, and the baby improved rapidly. In just under a month he was strong enough to go home.
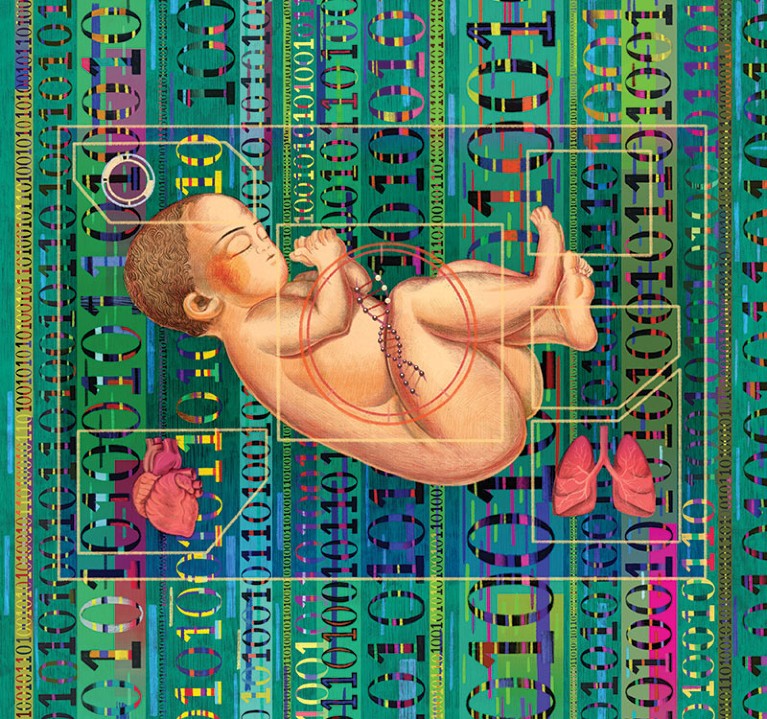
For children like Mitchell who are born with a genetic disease, it used to take years to get a diagnosis, and by then it often was too late. Now, however, advances in the speed of genetic sequencing and steeply falling costs have made it possible to screen for hundreds or even thousands of childhood-onset genetic diseases. Within the past year or so a few dozen hospitals have started offering the ability to rapidly sequence a newborn’s genome to help diagnose a life-threatening condition soon after birth. Researchers are studying whether such sequencing should be offered to all newborns as part of standard health screening. And companies such as Sema4 and BabyGenes are now marketing 23andMe-style direct-to-consumer tests to parents simply seeking to know more about the health of their baby. Prenatal and newborn genetic sequencing is expected to grow to an $11.2-billion industry by 2027, up from a $4-billion market in 2018.
Proponents say that genetic testing of newborns can help diagnose a life-threatening childhood-onset disease in urgent cases and could dramatically increase the number of genetic conditions all babies are screened for at birth, enabling earlier diagnosis and treatment. It could also inform parents of conditions they could pass on to future children or of their own risk of adult-onset diseases. Genetic testing could detect hundreds or even thousands of diseases, an order of magnitude more than current heel-stick blood tests—which all babies born in the U.S. undergo at birth—or confirm results from such a test.
Innovations In The DNA Drug Revolution
But others caution that genetic tests may do more harm than good. They could miss some diseases that heel-stick testing can detect and produce false positives for others, causing anxiety and leading to unnecessary follow-up testing. Sequencing children’s DNA also raises issues of consent and the prospect of genetic discrimination.
Regardless of these concerns, newborn genetic testing is already here, and it is likely to become only more common. But is the technology sophisticated enough to be truly useful for most babies? And are families—and society—ready for that information?
In the 1960s microbiologist Robert Guthrie developed a test for phenylketonuria (PKU), a genetic disorder that causes the amino acid phenylalanine to build up in the body. PKU is easily treated with a phenylalanine-restricted diet, but without intervention it can cause brain damage and mental disabilities. Within a few years other U.S. states required that Guthrie’s test be administered to newborns, and tests for other conditions were soon to follow. By the mid-1980s most states had mandatory screening programs. In 2002 the federal government asked the American College of Medical Genetics to develop guidelines for newborn screening, which culminated in the Recommended Universal Screening Panel, a set of 35 core conditions and 25 secondary ones that are treatable. Most states now test for a subset of these conditions.
There are roughly 14,000 known genetic diseases in humans, ranging from childhood-onset diseases such as PKU and congenital heart disease to adult-onset conditions such as Huntington’s disease and heritable forms of cancer. Some childhood diseases, such as PKU, are treatable if caught early. Heel-stick tests look for only a tiny fraction of these diseases, hence the appeal of genetic testing.
In the early 2010s researchers at the National Institute of Child Health and Human Development and the National Human Genome Research Institute launched a program, called NSIGHT (short for Newborn Sequencing in Genomic Medicine and Public Health), to explore the risks and benefits of DNA screening of newborns. Rady’s Kingsmore led one of four projects funded by NSIGHT, which explored the use of rapid, whole-genome sequencing in extremely sick newborns suspected of having a genetic disease.
Standard sequencing can take weeks, but using a rapid sequencing method and software that compared the genome with the patient’s disease characteristics, Kingsmore’s team could get a genetic diagnosis back in as little as a day or two. For these babies, hours or days can be the difference between life and death or severe disability. The first of two trials led by Kingsmore took place from 2014 to 2016 at Children’s Mercy Hospital in Kansas City. The second ran from 2017 to 2019 at Rady Children’s. Within the past year the group has started offering newborn sequencing at 23 hospitals around the country, and lawmakers from California have introduced federal legislation to cover the cost of sequencing critically ill babies through Medicaid. As of last November, Kingsmore and his colleagues had sequenced more than 1,100 babies with suspected genetic diseases. About one in three of them received a diagnosis that identified an illness, and one in four had their existing treatment changed as a result.
Mitchell Gorby was one of those sequenced at Rady (but not as part of NSIGHT). Carroll, the Rady neonatologist, says the information “helped us more confidently give him more transfusions and hold off on other testing.” It is possible Mitchell may have survived and outgrown his disorder without the test and diagnosis. But in other cases, sequencing has very likely saved lives. Moreover, sequencing probably significantly reduced the diagnostic odyssey such children have to take, Kingsmore says.

Extremely sick babies are not the only ones who could benefit from genetic testing. Another NSIGHT project investigated whether sequencing could also be used in clinical settings to screen newborns with no obvious signs of disease.
For this study, called the BabySeq Project, Robert Green of Brigham and Women’s Hospital, Alan Beggs of Harvard Medical School and their colleagues recruited families and randomly assigned half of them to have their babies’ genomes sequenced. They developed a list of about 1,500 genes that were highly associated with diseases that begin in childhood or adolescence, then returned information about a subset of those genes to the families. The goal was to do the most comprehensive testing possible—to see anything and everything that could be discovered about gene-based risks. Last January the group reported sequencing results from 159 newborns—mostly healthy babies but also some ill ones in the neonatal ICU. The scientists found that 9.4 percent of the healthy group were at risk of developing a childhood-onset disease that was not known from their medical or family history, and 88 percent were carriers for recessive diseases.
So was the testing worth it for parents? A mother named Natalie, who requested we use only her first name out of concern for her family’s privacy, has a son who was enrolled in BabySeq. Natalie, who is a physician and lives in Washington, D.C., admits she felt some nervousness about the testing. “Whenever you have the chance to learn about the health of your child, there’s an opportunity for anxiety,” she says. But overall, she and her husband were comfortable with the project. “Because they were looking at only genetic defects that affect childhood and only illnesses that had some preventive measures, we felt it could potentially be useful,” she says.
Fortunately, the results of tests on her son, Russell, did not turn up any childhood-onset genetic disorders. The exams did indicate that he may be a carrier for a recessive metabolic disorder called Gaucher disease, but the sequencing of this gene is particularly prone to error, so he will need follow-up testing to confirm. For other families, the benefits of sequencing were more clear-cut: one child had a disorder—missed by standard screening—that makes the body unable to recycle a vitamin called biotin; the condition can cause coma and death if left untreated, but it can easily be treated by supplementation.
Although BabySeq was initially focused only on childhood-onset disorders, one baby in the study was found to carry a variant of the BRCA2 gene, which is associated with a high risk of breast and other cancers, so the researchers asked parents for permission to inform them of the risk of adult-onset disorders if they chose. Natalie and her husband opted not to receive this information but said they would leave it up to Russell if he wanted to be tested when he was older. “We felt it should be our son’s decision,” Natalie says.
Because of its complexity and cost, BabySeq was never intended to be a feasible addition to standard newborn screening. “We have not tried to advocate for this in clinical practice,” Green of Brigham and Women’s says. But sequencing tests are no longer confined to clinical practice. Several companies now offer direct-to-consumer DNA tests for newborns. The firm Sema4 sells a test for $379 that it says screens for more than 190 genetic conditions that can occur before the age of 10 and that can be treated with medication, diet or other interventions. The company gives results to parents in a genetic-counseling session about four to six weeks after the test. Sema4’s CEO, Eric Schadt, says the test can detect disease-related genetic variants with 99 percent accuracy. Sema4 only reports results for diseases that have a greater than 80 percent penetrance—the proportion of people with a genetic variant who end up developing the disease. It also discloses information about the child’s sensitivity to certain drugs, although the U.S. Food and Drug Administration has recently been pressuring companies not to make such information available, because it says that it has not reviewed the tests and that they may not be backed up by clinical evidence.
Another company, BabyGenes, offers a test that scours 100 genes for more than 72 conditions. It is offered in the form of either a cheek swab or dried-blood spot test and retails for $349.
Schadt admits Sema4 doesn’t know whether the kind of testing it offers leads to an overall benefit for patients, although he says the company is doing studies to find out. There are reasons to wonder. The accuracy of these tests in detecting disease is still uncertain. In a third NSIGHT project, led by Jennifer Puck, Barbara Koenig and Pui-Yan Kwok of the University of California, San Francisco, researchers sequenced the DNA of dried spots of blood left over from newborn heel-stick tests (California has kept all its blood spots since the early 1980s). Although the sequencing did detect some genetic conditions that the standard newborn screening panel does not test for, it missed some of those that standard screening caught. And it flagged a lot of genetic variants of unknown significance, Puck says: “Newborn screening is very different from having a sick individual in front of you for whom you’re trying to arrive at a diagnosis.”
When combined with the standard screening, DNA testing did reduce the number of false positives, however. Puck thinks sequencing could be an add-on to standard screening when there’s an abnormal result, but she doesn’t think it should be used to screen all healthy babies. “We’re just not at the point where we can interpret the sequence with sufficient predictive value to say ‘yes’ or ‘no,’ this is a disease or not,” she says.
Another issue that concerns physicians and medical ethicists is the possibility that genetic testing will cause unnecessary anxiety for parents about diseases that may appear later in life or never show up at all. “When it comes to genetic information about your child, a lot of people aren’t in a position to well interpret what the results mean,” says Nita Farahany, a professor of law and philosophy at Duke University School of Law, who is an expert in genetics and bioethics. “If they’re told their child has a four times greater risk [of some condition], but the population risk is 1 percent, how do they treat their children?” There is already a shortage of genetic counselors in the U.S., so there would not be enough people to help parents understand their child’s genetic results.
Then there’s the issue of privacy. If the child’s genetic information is stored on file, who has access to it? If the information becomes public, it could lead to discrimination by employers or insurance companies. The Genetic Information Nondiscrimination Act (GINA), passed in 2008, prohibits such discrimination. But GINA does not apply to employers with fewer than 15 employees and does not cover insurance for long-term care, life or disability. It also does not apply to people employed and insured by the military’s Tricare system, such as Rylan Gorby. When his son’s genome was sequenced, researchers also obtained permission to sequence Rylan’s genome, to determine if he was a carrier for the rare hemoglobin condition. Because it manifests itself only in childhood, Gorby decided taking the test was worth the risk of possible discrimination.
Cost is another consideration. Clinical sequencing is still about $500 to $800, and interpretation can be upward of $1,000, according to Brigham and Women’s Green. For families who can’t afford health insurance, this is out of reach. Some experts have also raised concerns that genetic testing could lead to a lot of follow-up testing with specialists, which could overburden an already resource-strapped health care system. If sequencing turns out to save money in the long run, insurance companies may cover it, but there’s no guarantee.
Yet another problem is that the majority of the sequencing to date has been done in babies whose families are well-off and white, raising concerns that this could become the province of only the privileged. And the racial homogeneity could skew the results: diseases more prevalent in Caucasian individuals could be overrepresented in test panels, whereas illnesses more common in racial minorities may be underrepresented. (New medical data projects intend to address this disparity [see “All of Us].)
The U.C.S.F. NSIGHT project included a working group that investigated some of these ethical and policy issues, which culminated in a 2018 report by the Hastings Center, a bioethics nonprofit in Garrison, N.Y. The report concluded that newborn sequencing has many benefits in helping diagnose sick babies and could expand the number of conditions that meet the stringent newborn screening criteria. But using genome sequencing as a replacement for newborn screening is “at best premature,” the authors say, and direct-to-consumer sequencing should not be used for diagnosis or screening purposes.
Barbara Koenig, a professor of medical anthropology and bioethics at U.C.S.F. and one of the report’s co-authors, underscores the fact that sequencing, while promising, is not yet mature enough to be routinely used to screen healthy children. “This is not a technology that’s ready for prime time for use in healthy infants,” Koenig says.
Despite these concerns, the era of newborn sequencing is now upon us, and the practice will likely become more widespread as costs come down and the results become more accurate and useful. In the meantime, the risks and benefits of sequencing must be weighed on an individual basis. Extremely sick newborns are a completely different case from apparently healthy children of worried parents susceptible to marketing from genetic-testing firms.
For Mitchell Gorby, sequencing was certainly worth it. Two months after leaving the hospital, he is doing fine and has doubled his weight. His parents are settling into their new routine, somewhat sleep-deprived, but happy to be home with their healthy baby boy.

 Curing What Ails Us
Curing What Ails Us
 The Power of Spheres
The Power of Spheres
 Gene Therapy Arrives
Gene Therapy Arrives
 All of Us
All of Us