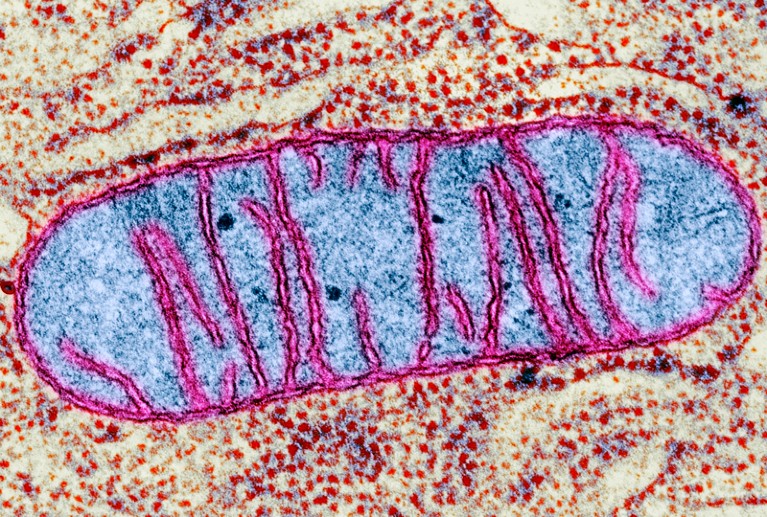
A mitochondrion, as seen through a transmission electron microscope. The membranes (in pink) prevent CRISPR–Cas9 genome editing.Credit: CNRI/SPL
Microbiologist Joseph Mougous has plenty of reasons to study microscopic warfare. Understanding inter-bacterial conflict, for example, can help researchers to learn why some microbes make animals and humans ill. But, as so often in research, his attempts to understand one set of problems have led to a tool for something different.
Mougous, at the University of Washington in Seattle, and his colleagues have described how an exceptional enzyme enabled them to edit the genomes of mitochondria, crucial energy-generating structures in many cells (B. Y. Mok et al. Nature http://doi.org/d3gd; 2020).
It’s a great achievement — even more so because the team had no such plans when it began the work. As the COVID‑19 pandemic forces funding agencies to reassess their priorities, it signals the value of foundational research.
Read the paper: A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing
The discovery is important because mutations in mitochondrial DNA can result in devastating disorders, such as Leber’s hereditary optic neuropathy, which causes vision loss and can lead to muscle weakness and heart problems. These could potentially be treated with genome editing, but current methods (including CRISPR–Cas9) can’t make precise changes to mitochondrial genomes, because crucial components can’t get into membrane-bound mitochondria.
Mougous and his team originally wanted to understand how bacteria deploy toxins to battle one another, and what impact this might have on bacterial ecosystems. They needed a toxin that could leave a trace, so they decided to look for one that can change the DNA of organisms it attacks.
They chose to follow an enzyme called DddA. When attacking a cell, DddA changes the DNA base C to U, which the cell’s own DNA-replication machinery reads as a T. Mougous proposed that his team should search bacterial battlegrounds for traces of these C-to-T changes. This would be a sign that the toxin had shaped the bacterial ecosystem.
But the researchers found that DddA was unusual in that it modifies double-stranded DNA, whereas most enzymes of its kind modify only single strands. And they discovered that with a few tweaks, it could be used to edit the double-stranded mitochondrial genome.
Mitochondrial genome editing gets precise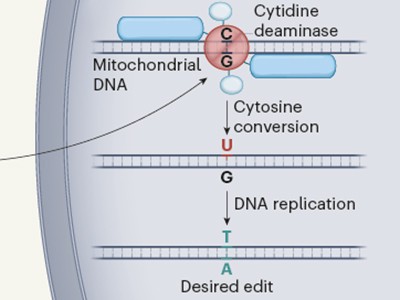
This discovery has the potential to treat human mitochondrial disorders, although it will need a great deal of further testing and refinement, as well as wide consultation and careful consideration of ethics, particularly for those applications that would create heritable genetic changes. It could give new energy to the search for antibiotics; many are derived from soil microbes, and editing the microbes’ genes could produce useful new chemicals. It could be used to edit animals’ mitochondria to generate models for studying diseases, which in turn could be deployed to seek treatments. And there are potential environmental and industrial applications, too. Microbial enzymes, for example, have been shown to degrade the widely used plastic polyethylene terephthalate (PET) — which is among the causes of plastic pollution — to its chemical building blocks. Gene editing could make them more effective. All from a discovery that, in its infancy, had little to do with such applications.
It won’t be the first time. CRISPR–Cas9, which is part of a rudimentary microbial immune system, was also discovered through curiosity-driven research. Scientists were interested in mysterious repeating sequences in the DNA of some microbes. Only later did they realize that the system could be harnessed to make directed changes to genomes — in fact almost any genome in which it has been tested.
Such discoveries offer a timely reminder for funders. As the COVID-19 pandemic wreaks havoc on economies and societies, and funds become scarce, many are understandably looking to reprioritize their spending. They have the unenviable task of balancing investments in basic and applied research. Both are important. Treatments and vaccines for COVID-19 underscore the importance of translational and clinical work. But one has only to look at the frenetic work being done to study the virus SARS-CoV-2 to see the importance of basic research, too.

 Scientists make precise gene edits to mitochondrial DNA for first time
Scientists make precise gene edits to mitochondrial DNA for first time
 Mitochondrial genome editing gets precise
Mitochondrial genome editing gets precise
 Read the paper: A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing
Read the paper: A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing