“Neurodegenerative diseases represent a huge societal burden and a tremendous opportunity to innovate and improve treatments, especially considering recent scientific breakthroughs in the field,” said Min Li, founder and CEO of SciNeuro Pharmaceuticals. As a clinical stage company, SciNeuro is focused on developing precision medicine therapies to treat a wide spectrum of neurodegenerative conditions, including Alzheimer’s disease (AD) and Parkinson’s disease (PD).
Based in Rockville, Maryland, United States, SciNeuro was launched in 2020 with a $100 million series A (early-stage) venture round co-led by ARCH Venture Partners and Lilly Asia Ventures. The company is led by Li, a neuroscientist and former global head of neuroscience research and development (R&D) at GSK, a multinational biopharma firm.
SciNeuro is focused on developing drugs on three key disease pathways—neurovascular inflammation, proteinopathy and immune response. “Focusing on the fundamental mechanisms involved in neurodegenerative processes provides us with targets that have multi-indication potential,” said Li. Of the company’s eight pipeline assets, the most advanced, a first-in-class therapy named SNP318, has already entered phase 1 clinical trials positioned for AD patients with neurovascular deficits, including those who are excluded from current anti-amyloid therapies.
Localized neurovascular inflammation is common in neurodegenerative diseases such as AD, and leads to reduced blood flow to the brain, leaky blood–brain barriers (BBB), and ultimately neuronal death. Neurovascular dysfunction represents the earliest and strongest brain pathology associated with AD1. SNP318 is a small molecule inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2) that reduces vascular inflammation to protect brain cells. SNP318 is a brain-penetrating Lp-PLA2 inhibitor that the company in-licensed from GSK. An earlier placebo-controlled phase 2a study conducted by GSK with a first-generation partial inhibitor of Lp-PLA2 demonstrated improvements in executive function, working memory, and key biomarkers in AD patients with neurovascular pathology2.
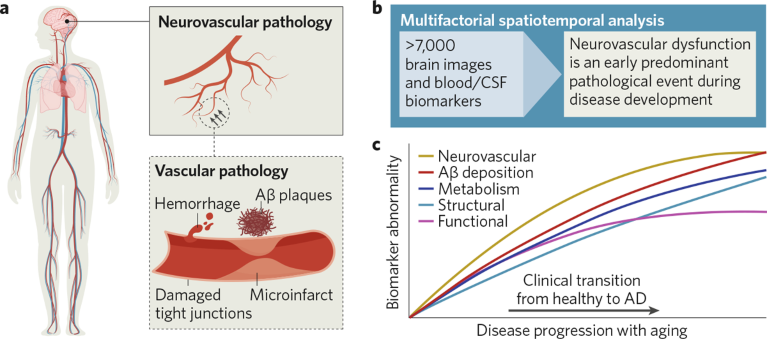
Targeting Alzheimer’s disease (AD). The human vascular system has an extensive brain vessel network or neurovascular network. a, Neurovascular pathology in AD includes Aβ plaque (β-amyloid deposits), microbleeds, microinfarcts and damaged tight junctions. b, Multifactorial data-driven analysis of large datasets from The Alzheimer’s Disease Neuroimaging Initiative (ADNI) has shown that neurovascular dysfunction is the earliest and predominant event among five key factors. c, Graphic representation of multifactorial spatiotemporal analysis adapted from figure 3 of the published paper1. CSF, cerebrospinal fluid.
Emerging clinical rationale
Recent SciNeuro phase 1 data have shown that SNP318 is significantly more potent than earlier Lp-PLA2 inhibitors and is able to efficiently penetrate the central nervous system, allowing it to achieve superior target engagement and broader anti-inflammatory benefits. The company expects to initiate a global phase 2 study in 2024.
SciNeuro is excited about the potential to treat AD through neurovascular protection and repair. Until very recently, AD therapies were unable to slow down disease progression. Recent United States Food and Drug Administration (FDA) approvals of anti-amyloid therapies offer new hope. “However, these therapies target only one aspect of AD’s complex pathobiology. Additionally, they can cause side effects, particularly in those with coexisting or AD-related cerebrovascular pathology,” said Sharon Cohen, neurologist and medical director of Toronto Memory Program, who has led multiple AD trials. “Improving neurovascular integrity and BBB function in AD are important targets and may offer additive or synergistic treatment benefits in patients with AD. Furthermore, improving vascular integrity has the potential to reduce the main side effect of anti-amyloid therapy, namely amyloid-related imaging abnormality (ARIA), which results from amyloid deposits within cerebral vessels referred to as cerebral amyloid angiopathy (CAA). Finally, individuals with significant CAA, for example, people with more than four microbleeds, are not eligible for anti-amyloid monoclonal antibodies, leaving a critical need for alternative therapeutic approaches,” said Cohen. Of particular interest, Lp-PLA2 inhibition in previous human studies has demonstrated BBB protection. Therefore, SNP318 affords the possibility to develop a complementary or alternative therapy.
Rebalancing immune function
Immune response is another core focus of SciNeuro’s pipeline with the goal of restoring a balanced, protective immune function to slow disease progression. The company’s lead program targets the leucine-rich repeat kinase 2 (LRRK2), a multifunctional kinase that is overly active in PD. Leveraging these disease biology insights, the company is developing an antisense oligonucleotide (ASO), SNP614, to reduce overall LRRK2 protein levels to treat PD. Recent preclinical studies have yielded highly encouraging results. A human study is being planned for 2024.
With multiple data readouts over the next 12 months in all three core areas, SciNeuro is on the cusp of growth by broadening its investor base and establishing new partnerships. “To achieve our goals, in addition to ongoing effective execution to advance the pipeline, we recognize the value of strategic partnerships, particularly those where the partner brings global reach and commitment to neurodegenerative diseases,” said Li.
Li hopes that within the next five years the company will fulfil the promise of its current pipeline with clinical validation. “SciNeuro has a robust and differentiated portfolio. These programs offer both mono and combo disease modifying treatment options. By continuing effective execution, we are optimistic that we will deliver a profound impact on the treatment paradigm of neurodegenerative diseases.”


