Getting drugs from the bloodstream into the brain is notoriously difficult because of the presence of the blood–brain barrier (BBB), a selectively permeable layer of cells that shield the brain from toxic substances. Overcoming this obstacle is crucial for treating a wide range of central nervous system (CNS)-related conditions.
“Biotherapeutics have revolutionized how we treat life-threatening diseases, but they can’t enter the brain,” said Mathias Schmidt, president and CEO of JCR USA. “Over the past 15 years, JCR has invested in basic research to develop a platform technology that transports biologics into the CNS.”
JCR Pharmaceuticals has been developing cutting-edge therapies for rare diseases since 1975. Founded in Hyogo, Japan, by Shin Ashida, the company now has over 900 employees in offices in Japan, the USA, Brazil and Europe.
JCR’s proprietary J-Brain Cargo technology relies on a transferrin receptor-targeting antibody to deliver effector proteins into the CNS (Fig. 1). By binding to transferrin receptors on BBB endothelial cells, J-Brain Cargo ferries drug payloads into the brain through a process called receptor-mediated transcytosis.
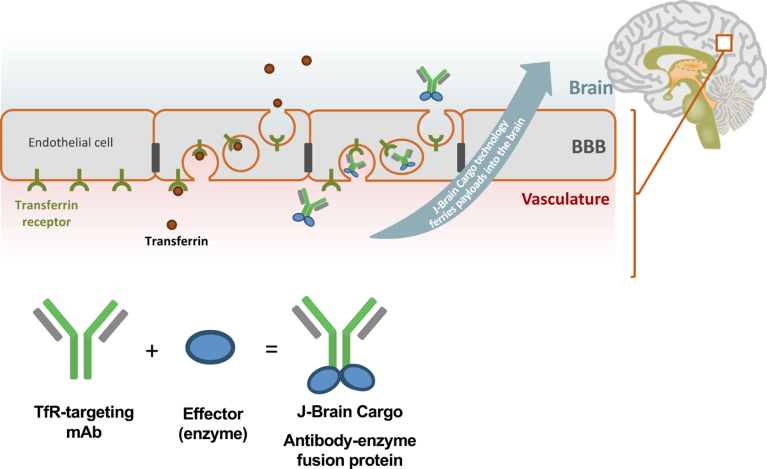
Fig. 1 | Mechanism of action of J-Brain Cargo technology to bring biotherapeutics across the BBB. The technology delivers effector proteins into the CNS, by fusing them to a transferrin receptor-targeting antibody which crosses the BBB by receptor-mediated transcytosis. BBB, blood–brain barrier; mAb, monoclonal antibody; TfR, transferrin receptor.
“We have copied the mechanism that mother nature invented to get larger molecules such as insulin and transferrin into the CNS,” Schmidt explained. “Our antibody binds to the transferrin receptor and crosses the BBB just like its natural ligand, without interfering with the receptor’s functionality.”
Schmidt compared J-Brain Cargo to a rocket that can bring satellites (biologic drugs) into the CNS orbit, where they can exert their therapeutic effect. JCR has been focusing on fusing therapeutic enzymes with J-Brain Cargo to address the CNS burden of lysosomal storage diseases (LSDs), for which no treatments were available until now.
A first-to-market BBB-crossing biologic
In March 2021, the first therapy using this technology, pabinafusp alfa, was approved in Japan for the treatment of mucopolysaccharidosis II (MPS II or Hunter syndrome).
MPS II is a rare, inherited disorder caused by defects in iduronate-2-sulfatase (IDS), an enzyme that breaks down complex sugars such as heparan and dermatan sulfate. Accumulation of these sugars in cells over time affects multiple tissues and organs. Although the clinical manifestations vary widely in severity, patients often display developmental delay and cognitive impairment in early childhood that progressively worsens.
Intravenous enzyme replacement therapy (ERT) with the recombinant human IDS (idursulfase) is a well-established treatment for MPS II. While ERT can alleviate some of the peripheral symptoms, it does not address the neurological symptoms and CNS complications.
Pabinafusp alfa is a recombinant fusion protein composed of idursulfase and J-Brain Cargo that is administered intravenously once a week. “I believe that pabinafusp alfa is changing the lives of individuals affected by the disease and their caregivers,” Schmidt said. “Surprisingly, we are finding in mouse models that the technology not only ameliorates the effects of disease on the brain, but also on other organs and tissues such as the retina, spinal cord and muscle.”
A clinical trial in 28 Japanese patients with MPS II demonstrated that after 52 weeks, pabinafusp alfa significantly reduced heparan sulfate concentrations in the cerebrospinal fluid and had a positive effect on neurocognitive development1. No serious treatment-related adverse events were reported. In light of these results, JCR has launched a global phase 3 trial.
“In extension and follow-up studies in Japan, we are observing improvements in daily-living skills and behavior, for example, that are not captured in clinical trials,” said Schmidt. “In a survey, we found that 100% of parents wanted their children to stay on the drug.” JCR is in the process of publishing these findings.
MPS II is just one of around 50 LSDs. JCR is harnessing its J-Brain Cargo technology platform to progress an industry-leading portfolio for the treatment of LSDs including MPS I, Sanfilippo syndrome, Tay–Sachs disease, fucosidosis, Pompe disease and Krabbe disease. The company has 17 programs in development, three of which are in clinical stages and six in pre-clinical stages.
“The pre-clinical data in Sanfilippo syndrome A are highly encouraging and our ongoing trial recruitment is already oversubscribed, highlighting the urgent need for treatment options in diseases with no established standard of care,” Schmidt added.
Going beyond LSDs
J-Brain Cargo technology is applicable for the treatment of many CNS disorders, and JCR is interested in partnering with companies focusing on therapies for neurodegenerative and other devastating neurological diseases. Recently, JCR announced collaboration agreements with Alexion and Angelini Pharma to develop new treatments using J-Brain Cargo technology for a neurodegenerative disease and epilepsy, respectively.
“We remain committed to providing transformative therapies to patients with LSDs but are keen to tap into other disease areas by establishing synergistic partnerships with companies seeking to progress the development of their CNS drugs,” Schmidt concluded.


