Cancer is the second leading cause of death in the United States and 40% of people will develop cancer in their lifetime. A simple blood test to detect cancer and assist oncologists in diagnosing disease in patients could help to save and improve lives.
Creatv Bio (Creatv) has developed a blood test with a wide spectrum of clinical applications that is built on the discovery of a cancer-generated cellular biomarker in the blood called cancer-associated macrophage-like cells (CAMLs) (Fig. 1)1.
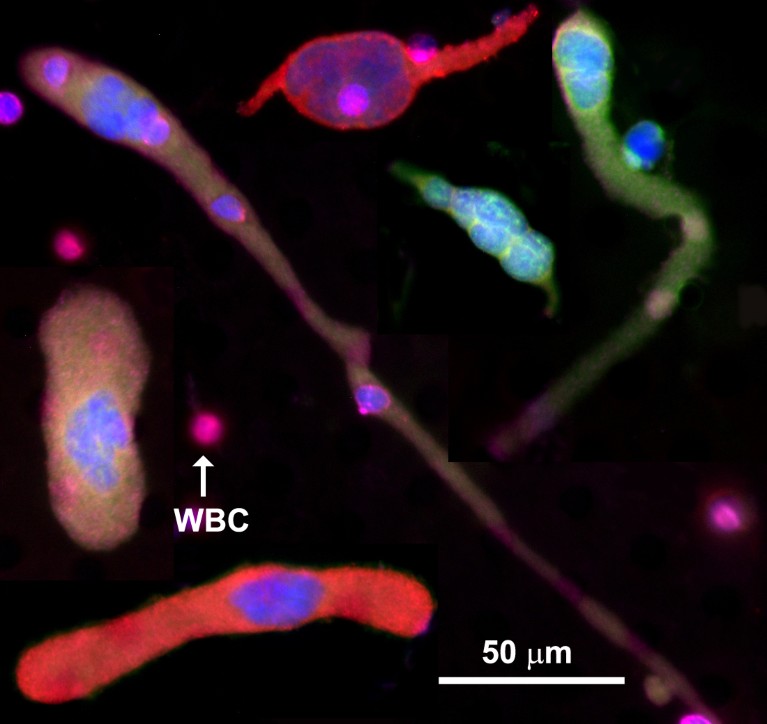
Fig. 1 | Cancer-associated macrophage-like cells (CAMLs) shown using fluorescent stains. Even though CAMLs have different morphologies, they have common features: a single tail or two tails on the opposite sides of the cell, and rod, oval or round shapes. CAMLs are polyploid. Also shown are the fluorescent stains DAPI (blue), CK8, 18, 19 (green), PD-L1 (red), and CD45 (purple). Other companion diagnostic markers can replace PD-L1 in the red channel. PD-L1, programmed cell death ligand 1; WBC, white blood cell. The scale is 50 µm.
Creatv’s blood test isolates both CAMLs and circulating tumor cells (CTCs), the latter of which are tumor cells that have dislodged from primary cancers and entered the blood circulation. CAMLs are more prevalent than CTCs and can be found in all cancer subtypes and in all stages of disease, whereas CTCs are usually found in metastatic breast, prostate, or colorectal cancers. The polynucleated nature of CAMLs results from their growth in size when they engulf tumor cells, which Creatv discovered more than ten years ago1.
Research by Creatv has led to the development of the LifeTracDx liquid biopsy, applicable to both cancer screening and cancer diagnostics. Creatv’s blood test capabilities for cancer screening will be described in another article. The four clinical diagnostic applications, explained below, reduce the need for tissue biopsies, improve cancer care and assist pharmaceuticals in developing novel drugs2.
Predicting treatment response
Physicians rely on imaging to monitor whether a cancer patient is responding to a therapy, but it can take months using classical imaging methodologies to visualize if a tumor has shrunk or grown. Accelerating the assessment of the tumor response could enable doctors to switch non-responding patients to potentially more effective therapies, reducing the time patients spend taking drugs that fail to shrink tumors.
Early clinical trials suggest that CAMLs can predict treatment response as early as 30 days after administration of the first dose of new therapies3,4. The ability to predict treatment response could help a physician to improve patient care and could aid drug companies to rapidly evaluate the efficacy of their therapy compounds.
Assessing a patient’s prognosis
The features of a patient’s CAMLs before the start of treatment have been shown to offer insights into their prognosis. Creatv analyses six primary features of CAMLs that are characteristic of highly aggressive tumors. CAML size is one of the features. For example, if a CAML is larger than 50 µm at baseline, a patient is likely to have an aggressive tumor and poorer prognosis. Creatv’s work, in collaboration with internationally renowned hospitals, suggests that smaller CAMLs are associated with better survival in more than 20 types of tumor. Other indicators of aggressive cancer include the number of CAMLs, presence of dividing cells, and micronuclei development inside CAMLs, and other features, which have been described as indicators of aggressive cancer5,6,7,8,9,10,11,12.
Companion diagnostics
As cancer treatment becomes more targeted, physicians have begun to use companion diagnostics to pre-determine whether a patient may benefit from a subtype of therapy. These tests use biomarkers present in or on the surface of the tumor to determine targeted drug efficacy.
Some companion diagnostics require tissue biopsies, such as immunotherapy13. Taking tissue samples is expensive and can carry a risk to the patient. LifeTracDx blood tests are a cost effective, safe way to determine whether a patient may benefit from a drug. Creatv has developed more than 30 different companion diagnostics and actively collaborates with drug companies to develop novel companion diagnostics14,15.
Monitoring therapy
Disease can progress in many patients with cancer even after a positive initial response to therapy because of sub-clonal resistance mutations that drive the growth of treatment resistance cell proliferation. Disease progression is typically detected by imaging, which requires the tumor to grow. However, analyzing CAMLs in blood samples may accelerate the detection of tumor resistance, which could identify disease progression months ahead of what can be detected through imaging alone.
Conclusion
Creatv is in the process of opening a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory in New Jersey, United States, to provide the LifeTracDx blood test to assist physicians in their care for patients with cancer and enable the detection of disease before the onset of clinical symptoms. Creatv is actively looking for collaborators to evaluate a variety of clinical applications and is also actively helping pharmaceutical partners to develop cancer drugs. Creatv is addressing long-standing limitations of cancer diagnostics by imaging and aims to provide oncologists with timely insights into health and treatment options for patients.


