Abstract
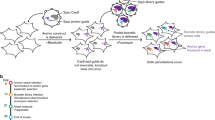
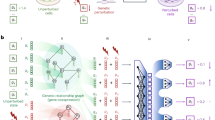
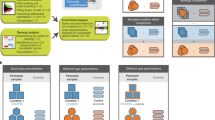
Understanding the direction of information flow is essential for characterizing how genetic networks affect phenotypes. However, methods to find genetic interactions largely fail to reveal directional dependencies. We combine two orthogonal Cas9 proteins from Streptococcus pyogenes and Staphylococcus aureus to carry out a dual screen in which one gene is activated while a second gene is deleted in the same cell. We analyze the quantitative effects of activation and knockout to calculate genetic interaction and directionality scores for each gene pair. Based on the results from over 100,000 perturbed gene pairs, we reconstruct a directional dependency network for human K562 leukemia cells and demonstrate how our approach allows the determination of directionality in activating genetic interactions. Our interaction network connects previously uncharacterized genes to well-studied pathways and identifies targets relevant for therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Beltrao, P., Cagney, G. & Krogan, N.J. Quantitative genetic interactions reveal biological modularity. Cell 141, 739–745 (2010).
Fischer, B. et al. A map of directional genetic interactions in a metazoan cell. eLife 4 (2015).
Drees, B.L. et al. Derivation of genetic interaction networks from quantitative phenotype data. Genome Biol. 6, R38 (2005).
St. Onge, R.P. et al. Systematic pathway analysis using high-resolution fitness profiling of combinatorial gene deletions. Nat. Genet. 39, 199–206 (2007).
Avery, L. & Wasserman, S. Ordering gene function: the interpretation of epistasis in regulatory hierarchies. TIG 8, 312–316 (1992).
Costanzo, M. et al. A global genetic interaction network maps a wiring diagram of cellular function. Science 353, aaf1420 (2016).
Wright, A.V., Nuñez, J.K. & Doudna, J.A. Biology and applications of CRISPR systems: harnessing nature's toolbox for genome engineering. Cell 164, 29–44 (2016).
Shalem, O., Sanjana, N.E. & Zhang, F. High-throughput functional genomics using CRISPR-Cas9. Nat. Rev. Genet. 16, 299–311 (2015).
Boettcher, M. & McManus, M.T. Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol. Cell 58, 575–585 (2015).
O'Hare, T., Deininger, M.W., Eide, C.A., Clackson, T. & Druker, B.J. Targeting the BCR-ABL signaling pathway in therapy-resistant Philadelphia chromosome-positive leukemia. Clin. Cancer Res. 17, 212–221 (2011).
Tanenbaum, M.E., Gilbert, L.A., Qi, L.S., Weissman, J.S. & Vale, R.D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 159, 635–646 (2014).
Huang, W., Sherman, B.T. & Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Dhillon, A.S., Hagan, S., Rath, O. & Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 26, 3279–3290 (2007).
Milojkovic, D. & Apperley, J. Mechanisms of resistance to imatinib and second-generation tyrosine inhibitors in chronic myeloid leukemia. Clin. Cancer Res. 15, 7519–7527 (2009).
de Jong, R., ten Hoeve, J., Heisterkamp, N. & Groffen, J. Crkl is complexed with tyrosine-phosphorylated Cbl in Ph-positive leukemia. J. Biol. Chem. 270, 21468–21471 (1995).
Cilloni, D. & Saglio, G. Molecular pathways: BCR-ABL. Clin. Cancer Res. 18, 930–937 (2012).
Cong, F. et al. Cytoskeletal protein PSTPIP1 directs the PEST-type protein tyrosine phosphatase to the c-Abl kinase to mediate Abl dephosphorylation. Mol. Cell 6, 1413–1423 (2000).
Calvisi, D.F. et al. Inactivation of Ras GTPase-activating proteins promotes unrestrained activity of wild-type Ras in human liver cancer. J. Hepatol. 54, 311–319 (2011).
Kuo, T.C., Chavarria-Smith, J.E., Huang, D. & Schlissel, M.S. Forced expression of cyclin-dependent kinase 6 confers resistance of pro-B acute lymphocytic leukemia to Gleevec treatment. Mol. Cell. Biol. 31, 2566–2576 (2011).
Sherr, C.J., Beach, D. & Shapiro, G.I. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 6, 353–367 (2016).
Cheah, C.Y. et al. Patients with myeloid malignancies bearing PDGFRB fusion genes achieve durable long-term remissions with imatinib. Blood 123, 3574–3577 (2014).
Li, F. et al. FGFR-mediated reactivation of MAPK signaling attenuates antitumor effects of imatinib in gastrointestinal stromal tumors. Cancer Discov. 5, 438–451 (2015).
Chase, A. et al. Imatinib sensitivity as a consequence of a CSF1R-Y571D mutation and CSF1/CSF1R signaling abnormalities in the cell line GDM1. Leukemia 23, 358–364 (2009).
Dufies, M. et al. Mechanisms of AXL overexpression and function in Imatinib-resistant chronic myeloid leukemia cells. Oncotarget 2, 874–885 (2011).
Ran, F.A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Konermann, S. et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517, 583–588 (2015).
Qi, L.S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013).
Vojta, A. et al. Repurposing the CRISPR-Cas9 system for targeted DNA methylation. Nucleic Acids Res. 44, 5615–5628 (2016).
Dahlman, J.E. et al. Orthogonal gene knockout and activation with a catalytically active Cas9 nuclease. Nat. Biotechnol. 33, 1159–1161 (2015).
Stowe, I.B. et al. A shared molecular mechanism underlies the human rasopathies Legius syndrome and Neurofibromatosis-1. Genes Dev. 26, 1421–1426 (2012).
Graham, D.K., DeRyckere, D., Davies, K.D. & Earp, H.S. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat. Rev. Cancer 14, 769–785 (2014).
Gay, C.M., Balaji, K. & Byers, L.A. Giving AXL the axe: targeting AXL in human malignancy. Br. J. Cancer 116, 415–423 (2017).
Postel-Vinay, S. & Ashworth, A. AXL and acquired resistance to EGFR inhibitors. Nat. Genet. 44, 835–836 (2012).
Sheridan, C. First Axl inhibitor enters clinical trials. Nat. Biotechnol. 31, 775–776 (2013).
Li, Z. et al. The OncoPPi network of cancer-focused protein-protein interactions to inform biological insights and therapeutic strategies. Nat. Commun. 8, 14356 (2017).
Laufer, C., Fischer, B., Billmann, M., Huber, W. & Boutros, M. Mapping genetic interactions in human cancer cells with RNAi and multiparametric phenotyping. Nat. Methods 10, 427–431 (2013).
Roguev, A. et al. Quantitative genetic-interaction mapping in mammalian cells. Nat. Methods 10, 432–437 (2013).
Bassik, M.C. et al. A systematic mammalian genetic interaction map reveals pathways underlying ricin susceptibility. Cell 152, 909–922 (2013).
Vizeacoumar, F.J. et al. A negative genetic interaction map in isogenic cancer cell lines reveals cancer cell vulnerabilities. Mol. Syst. Biol. 9, 696 (2013).
Shen, J.P. et al. Combinatorial CRISPR-Cas9 screens for de novo mapping of genetic interactions. Nat. Methods 14, 573–576 (2017).
Han, K. et al. Synergistic drug combinations for cancer identified in a CRISPR screen for pairwise genetic interactions. Nat. Biotechnol. 35, 463–474 (2017).
Blomen, V.A. et al. Gene essentiality and synthetic lethality in haploid human cells. Science 350, 1092–1096 (2015).
Du, D. et al. Genetic interaction mapping in mammalian cells using CRISPR interference. Nat. Methods 14, 577–580 (2017).
Kampmann, M., Bassik, M.C. & Weissman, J.S. Integrated platform for genome-wide screening and construction of high-density genetic interaction maps in mammalian cells. Proc. Natl. Acad. Sci. USA 110, E2317–E2326 (2013).
Gilbert, L.A. et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647–661 (2014).
LaMontagne, K.R. Jr., Flint, A.J., Franza, B.R. Jr., Pandergast, A.M. & Tonks, N.K. Protein tyrosine phosphatase 1B antagonizes signalling by oncoprotein tyrosine kinase p210 bcr-abl in vivo. Mol. Cell. Biol. 18, 2965–2975 (1998).
Bae, S., Park, J. & Kim, J.S. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475 (2014).
Burk, O. & Klempnauer, K.H. Myb and Ets transcription factors cooperate at the myb-inducible promoter of the tom-1 gene. Biochim. Biophys. Acta 1446, 243–252 (1999).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 343, 84–87 (2014).
Acknowledgements
Special thanks go to members of the McManus Lab who provided critical feedback during the course of this project and E. Cahill for excellent technical support. We also thank L.A. Gilbert and M.E. Tanenbaum for sharing the CRISPRa cell line ahead of its publication. M.T.M. was supported by NIH/CTD2 (U01CA168370) and IDG (1U01MH105028). M.K. was supported by NIH/NIGMS New Innovator Award DP2 GM119139, NIH/NCI K99/R00 CA181494, a Stand Up to Cancer Innovative Research Grant and the Chan Zuckerberg Biohub. J.A.B. was supported by NIH Training grant T32 GM00715 and an AFPE Predoctoral Fellowship. H.F. was supported by NIH/CTD2 (U01CA168449).
Author information
Authors and Affiliations
Contributions
The project was conceived and directed by M.B. and M.T.M. Screen optimization was performed by M.B. and D.W. Libraries were designed by J.A.B. with guidance from M.B. and cloned by M.B. Orthogonal vectors and cell lines were created by M.B. All screens were performed by M.B., with A.B. assisting in CRISPRa screen analysis. R.T. developed the computational pipelines and the statistical framework for data analysis for screens with guidance from M.K. R.T. also selected the best-performing sgRNAs for arrayed validation. M.B. and R.T.W. conducted and analyzed arrayed validation experiments. E.M. and R.T.W. performed western blot analyses. X.M. and H.F. conducted and analyzed TR-FRET experiments. M.B. and M.T.M. wrote the manuscript with critical input from R.T., M.K., N.Z. and F.M. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 (PDF 12455 kb)
Supplementary Table 1
Read count values for each separate sgRNAs from primary CRISPRa screen together with sgRNA nucleotide sequences. (CSV 25544 kb)
Supplementary Table 2
Transcript level analysis of primary CRISPRa screen with τ and p-values as well as expression levels (FPKM) for each transcript are shown. Mann-Whitney U test was used to calculate p-values as described previously44. To correct for multiple hypothesis testing, we first performed random sampling with replacement among the set of values for nontargeting control sgRNAs and calculated p-values for each sampling. A total of 300 million cells and 26,718 transcripts were analysed. (CSV 1123 kb)
Supplementary Table 3
Arrayed validation of 20 candidate genes from primaryCRISPRa screen are shown. (CSV 10 kb)
Supplementary Table 4
Nucleotide sequences of sgRNAs used in the CRISPRaposition of the orthogonal library are shown together withnumbers of potential off-target sites as determined by CasOFFinder. (CSV 6 kb)
Supplementary Table 5
Nucleotide sequences for sgRNAs used in the SaCas9nuclease (knockout) position of the orthogonal library areshown. (CSV 568 kb)
Supplementary Table 6
For gene:gene combinations from the orthogonal screensingle activation and knockout τ values, expected andmeasured double perturbation τ values as well as calculated GI and Ψ scores are shown from each clonal replicate separately. (CSV 211 kb)
Supplementary Table 7
Reproducible gene:gene combinations that passed the filter criteria and that were used to construct the genetic interaction network in Figure 3h are shown. (CSV 14 kb)
Supplementary Table 8
Individual relative fitness values (τ) from arrayed validation of selected gene:gene interactions. Shown are enrichment values of cells expressing the indicated combination of sgRNAs in the absence (no drug) or presence of imatinib (IM) (PDF 40 kb)
Supplementary Table 9
GIv scores for each gene:gene combination and time point were calculated based on τ values in Extended Table 8. Correlation between GIv scores from each arrayed validation time point and the orthogonal screen in clonal replicate 2 are shown. For day 14 of the arrayed validation in the presence of imatinib, Ψ scores are shown which are also indicated in the genetic interaction network model in Figure 4c. (PDF 53 kb)
Supplementary Table 10
Orthogonal screen raw read counts from baseline and Day19 (imatinib treated) of clonal replicates 1 and 2. Dual sgRNA construct names are in the format: CRISPRa target gene symbol (two sgRNA per gene labelled gene-A and gene-B) followed by SaCas9 nuclease target gene symbol, RefSeq accession number and sgRNA sequence. All CRISPRa sgRNA sequences are shown in Extended Table 4. All SaCas9 nuclease sequences are shown in Extended Table 5. (XLS 162566 kb)
Supplementary Data 1
Map of sgLenti vector for CRISPRa screen sgRNAexpression. (TXT 20 kb)
Supplementary Data 2
Map sgLenti orthogonal vector for orthogonal screen sgRNA expression. (TXT 17 kb)
Supplementary Data 3
Map of S.aureus Cas9 nuclease vector. (TXT 22 kb)
Rights and permissions
About this article
Cite this article
Boettcher, M., Tian, R., Blau, J. et al. Dual gene activation and knockout screen reveals directional dependencies in genetic networks. Nat Biotechnol 36, 170–178 (2018). https://doi.org/10.1038/nbt.4062
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.4062
This article is cited by
-
Genome-wide screens identify SEL1L as an intracellular rheostat controlling collagen turnover
Nature Communications (2024)
-
Complex synthetic lethality in cancer
Nature Genetics (2023)
-
CRISPR-Combo–mediated orthogonal genome editing and transcriptional activation for plant breeding
Nature Protocols (2023)
-
Optimized metrics for orthogonal combinatorial CRISPR screens
Scientific Reports (2023)
-
Make it a Combo
Nature Plants (2022)