Abstract
NLRX1 is unique among the nucleotide-binding-domain and leucine-rich-repeat (NLR) proteins in its mitochondrial localization and ability to negatively regulate antiviral innate immunity dependent on the adaptors MAVS and STING. However, some studies have suggested a positive regulatory role for NLRX1 in inducing antiviral responses. We found that NLRX1 exerted opposing regulatory effects on viral activation of the transcription factors IRF1 and IRF3, which might potentially explain such contradictory results. Whereas NLRX1 suppressed MAVS-mediated activation of IRF3, it conversely facilitated virus-induced increases in IRF1 expression and thereby enhanced control of viral infection. NLRX1 had a minimal effect on the transcription of IRF1 mediated by the transcription factor NF-kB and regulated the abundance of IRF1 post-transcriptionally by preventing translational shutdown mediated by the double-stranded RNA (dsRNA)-activated kinase PKR and thereby allowed virus-induced increases in the abundance of IRF1 protein.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Guo, H., Callaway, J.B. & Ting, J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 21, 677–687 (2015).
Schneider, M. et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat. Immunol. 13, 823–831 (2012).
Xia, X. et al. NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity 34, 843–853 (2011).
Benko, S., Magalhaes, J.G., Philpott, D.J. & Girardin, S.E. NLRC5 limits the activation of inflammatory pathways. J. Immunol. 185, 1681–1691 (2010).
Allen, I.C. et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity 36, 742–754 (2012).
Moore, C.B. et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 451, 573–577 (2008).
Allen, I.C. et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-κB signaling pathways. Immunity 34, 854–865 (2011).
Guo, H. et al. NLRX1 Sequesters STING to negatively regulate the interferon response, thereby facilitating the replication of HIV-1 and DNA viruses. Cell Host Microbe 19, 515–528 (2016).
Rebsamen, M. et al. NLRX1/NOD5 deficiency does not affect MAVS signalling. Cell Death Differ. 18, 1387 (2011).
Jaworska, J. et al. NLRX1 prevents mitochondrial induced apoptosis and enhances macrophage antiviral immunity by interacting with influenza virus PB1-F2 protein. Proc. Natl. Acad. Sci. USA 111, E2110–E2119 (2014).
Tattoli, I. et al. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-κB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 9, 293–300 (2008).
Qu, L. & Lemon, S.M. Hepatitis A and hepatitis C viruses: divergent infection outcomes marked by similarities in induction and evasion of interferon responses. Semin. Liver Dis. 30, 319–332 (2010).
Ikeda, M. et al. Human hepatocyte clonal cell lines that support persistent replication of hepatitis C virus. Virus Res. 56, 157–167 (1998).
Li, K., Chen, Z., Kato, N., Gale, M. Jr. & Lemon, S.M. Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-beta production in hepatocytes. J. Biol. Chem. 280, 16739–16747 (2005).
Yamane, D. et al. Differential hepatitis C virus RNA target site selection and host factor activities of naturally occurring miR-122 3´ variants. Nucleic Acids Res. 45, 4743–4755 (2017).
Feng, Z. et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 496, 367–371 (2013).
Abdul-Sater, A.A. et al. Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member NLRX1. J. Biol. Chem. 285, 41637–41645 (2010).
Lei, Y. et al. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity 36, 933–946 (2012).
Soares, F. et al. The mitochondrial protein NLRX1 controls the balance between extrinsic and intrinsic apoptosis. J. Biol. Chem. 289, 19317–19330 (2014).
Sumpter, R. Jr. et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79, 2689–2699 (2005).
Masuda, K. et al. Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proc. Natl. Acad. Sci. USA 110, 9409–9414 (2013).
Odendall, C. et al. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat. Immunol. 15, 717–726 (2014).
Hiscott, J. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 282, 15325–15329 (2007).
Mboko, W.P. et al. Interferon regulatory factor 1 restricts gammaherpesvirus replication in primary immune cells. J. Virol. 88, 6993–7004 (2014).
Fujita, T., Kimura, Y., Miyamoto, M., Barsoumian, E.L. & Taniguchi, T. Induction of endogenous IFN-alpha and IFN-beta genes by a regulatory transcription factor, IRF-1. Nature 337, 270–272 (1989).
Dauber, B. & Wolff, T. Activation of the antiviral kinase PKR and viral countermeasures. Viruses 1, 523–544 (2009).
Hong, M., Yoon, S.I. & Wilson, I.A. Structure and functional characterization of the RNA-binding element of the NLRX1 innate immune modulator. Immunity 36, 337–347 (2012).
Unger, B.L. et al. Nod-like receptor X-1 is required for rhinovirus-induced barrier dysfunction in airway epithelial cells. J. Virol. 88, 3705–3718 (2014).
Brown, E.A., Zajac, A.J. & Lemon, S.M. In vitro characterization of an internal ribosomal entry site (IRES) present within the 5′ nontranslated region of hepatitis A virus RNA: comparison with the IRES of encephalomyocarditis virus. J. Virol. 68, 1066–1074 (1994).
Barouch, D.H. et al. Rapid inflammasome activation following mucosal SIV infection of Rhesus monkeys. Cell 165, 656–667 (2016).
Soares, F. et al. NLRX1 does not inhibit MAVS-dependent antiviral signalling. Innate Immun. 19, 438–448 (2013).
Hirai-Yuki, A. et al. MAVS-dependent host species range and pathogenicity of human hepatitis A virus. Science 353, 1541–1545 (2016).
Yang, Y. et al. Disruption of innate immunity due to mitochondrial targeting of a picornaviral protease precursor. Proc. Natl. Acad. Sci. USA 104, 7253–7258 (2007).
Qu, L. et al. Disruption of TLR3 signaling due to cleavage of TRIF by the hepatitis A virus protease-polymerase processing intermediate, 3CD. PLoS Pathog. 7, e1002169 (2011).
Li, K. et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. USA 102, 2992–2997 (2005).
Li, X.D., Sun, L., Seth, R.B., Pineda, G. & Chen, Z.J. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc. Natl. Acad. Sci. USA 102, 17717–17722 (2005).
Honda, K. & Taniguchi, T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6, 644–658 (2006).
Ivashkiv, L.B. & Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 14, 36–49 (2014).
Tailor, P. et al. The feedback phase of type I interferon induction in dendritic cells requires interferon regulatory factor 8. Immunity 27, 228–239 (2007).
Li, P. et al. IRF8 and IRF3 cooperatively regulate rapid interferon-β induction in human blood monocytes. Blood 117, 2847–2854 (2011).
Lazear, H.M. et al. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog. 9, e1003118 (2013).
Weiss, G. et al. MyD88 drives the IFN-β response to Lactobacillus acidophilus in dendritic cells through a mechanism involving IRF1, IRF3, and IRF7. J. Immunol. 189, 2860–2868 (2012).
Ousman, S.S., Wang, J. & Campbell, I.L. Differential regulation of interferon regulatory factor (IRF)-7 and IRF-9 gene expression in the central nervous system during viral infection. J. Virol. 79, 7514–7527 (2005).
Scherbik, S.V., Stockman, B.M. & Brinton, M.A. Differential expression of interferon (IFN) regulatory factors and IFN-stimulated genes at early times after West Nile virus infection of mouse embryo fibroblasts. J. Virol. 81, 12005–12018 (2007).
Xu, H. et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat. Immunol. 13, 642–650 (2012).
Colina, R. et al. Translational control of the innate immune response through IRF-7. Nature 452, 323–328 (2008).
Lee, M.S., Kim, B., Oh, G.T. & Kim, Y.J. OASL1 inhibits translation of the type I interferon-regulating transcription factor IRF7. Nat. Immunol. 14, 346–355 (2013).
Elde, N.C., Child, S.J., Geballe, A.P. & Malik, H.S. Protein kinase R reveals an evolutionary model for defeating viral mimicry. Nature 457, 485–489 (2009).
Garaigorta, U. & Chisari, F.V. Hepatitis C virus blocks interferon effector function by inducing protein kinase R phosphorylation. Cell Host Microbe 6, 513–522 (2009).
Lu, B. et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488, 670–674 (2012).
Yamane, D. et al. Regulation of the hepatitis C virus RNA replicase by endogenous lipid peroxidation. Nat. Med. 20, 927–935 (2014).
Blight, K.J., McKeating, J.A. & Rice, C.M. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 76, 13001–13014 (2002).
Lemon, S.M. et al. Antigenic and genetic variation in cytopathic hepatitis A virus variants arising during persistent infection: evidence for genetic recombination. J. Virol. 65, 2056–2065 (1991).
Feng, Z. et al. Human pDCs preferentially sense enveloped hepatitis A virions. J. Clin. Invest. 125, 169–176 (2015).
Shimakami, T. et al. Protease inhibitor-resistant hepatitis C virus mutants with reduced fitness from impaired production of infectious virus. Gastroenterology 140, 667–675 (2011).
Yi, M. & Lemon, S.M. Replication of subgenomic hepatitis A virus RNAs expressing firefly luciferase is enhanced by mutations associated with adaptation of virus to growth in cultured cells. J. Virol. 76, 1171–1180 (2002).
Dansako, H. et al. Class A scavenger receptor 1 (MSR1) restricts hepatitis C virus replication by mediating toll-like receptor 3 recognition of viral RNAs produced in neighboring cells. PLoS Pathog. 9, e1003345 (2013).
Saeed, M. et al. SEC14L2 enables pan-genotype HCV replication in cell culture. Nature 524, 471–475 (2015).
Bryksin, A.V. & Matsumura, I. Overlap extension PCR cloning: a simple and reliable way to create recombinant plasmids. Biotechniques 48, 463–465 (2010).
Lanford, R.E. et al. Acute hepatitis A virus infection is associated with a limited type I interferon response and persistence of intrahepatic viral RNA. Proc. Natl. Acad. Sci. USA 108, 11223–11228 (2011).
Suzuki, K., Bose, P., Leong-Quong, R.Y., Fujita, D.J. & Riabowol, K. REAP: A two minute cell fractionation method. BMC Res. Notes 3, 294 (2010).
Li, Y., Masaki, T., Shimakami, T. & Lemon, S.M. hnRNP L and NF90 interact with hepatitis C virus 5′-terminal untranslated RNA and promote efficient replication. J. Virol. 88, 7199–7209 (2014).
Mo, J. et al. Pathogen sensing by nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is mediated by direct binding to muramyl dipeptide and ATP. J. Biol. Chem. 287, 23057–23067 (2012).
Wang, Q. et al. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J. Immunol. 183, 6989–6997 (2009).
Masaki, T. et al. miR-122 stimulates hepatitis C virus RNA synthesis by altering the balance of viral RNAs engaged in replication versus translation. Cell Host Microbe 17, 217–228 (2015).
Ziehr, B., Vincent, H.A. & Moorman, N.J. Human cytomegalovirus pTRS1 and pIRS1 antagonize protein kinase R to facilitate virus replication. J. Virol. 90, 3839–3848 (2016).
Schmidt, E.K., Clavarino, G., Ceppi, M. & Pierre, P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 6, 275–277 (2009).
Shimakami, T. et al. Stabilization of hepatitis C virus RNA by an Ago2-miR-122 complex. Proc. Natl. Acad. Sci. USA 109, 941–946 (2012).
Acknowledgements
We thank B. Glaunsinger (University of California, Berkeley) for the human IL6 3′ UTR expression vector, and L. Hensley, W. Lovell, M. Deng, and M. Chua for technical support. Supported by the US National Institutes of Health (U19-AI109965 to S.M.L., J.A.D. and J.P-Y.T.; R01-AI103083 to S.M.L.; R01-AI131685 to S.M.L. and J.K.W.; R01-AI103311 to N.J.M.; and R21-AI117575 to J.K.W.) and the University of North Carolina Cancer Research Fund (S.M.L. and J.P-Y.T.).
Author information
Authors and Affiliations
Contributions
H.F. and S.M.L. designed the experiments, analyzed the data and wrote the manuscript; H.F., E.M.L., D.Y., D.R.M., O.G -L. and I.M. performed experiments; E.W., J.M., H.G., L.M.R., J.K.W., J.P -Y.T., J.A.D. and N.J.M. provided critical reagents and intellectual input; S.M.L. supervised the study; and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
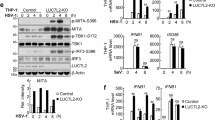
Supplementary Figure 1 The antiviral activity of NLRX1 results from positive regulation of cytokine responses.
(a) Kinetics of IL6 mRNA expression in NLRX1-deficient PH5CH8 cells infected with SeV. (b) Gaussia princeps luciferase (GLuc) analysis of HCV (JFH1-QL/GLuc) replication in NLRX1-T3 cells at 48 h post-transfection (hpt) with indicated treatment. NAC, N-acetyl-L-cysteine (3 mM); TUDCA, tauroursodeoxycholic acid (0.5 mM); 3-MA, 3-methyladenine (10 mM). Because basal levels of lipid peroxidation inhibit HCV replication, we used a lipid-insoluble antioxidant to avoid spurious increases in replication51. Viral RNA transfection and treatment with DAA or inhibitors were carried out as in Fig. 1b. (c) Transwell assay to assess the role of soluble cytokines in suppression of viral replication at 48 hpt. The upper wells were seeded with IRF1-deficient (indicator) PH5CH8 cells harboring a replicating HCV reporter virus genome (JFH1-QL/GLuc) that expresses GLuc, and placed in chambers in which the lower well was seeded with control versus NLRX1-deficient PH5CH8 cells stimulated by transfection with replicating HCV (JFH1-QL) RNA. (d) Replication of an HCV reporter virus assessed by GLuc expression at 48 hpt in control PH5CH8 cells versus NLRX1-T3 cells, both treated with ruxolitinib (3 μM, an inhibitor of JAK1 and JAK2), tofacitinib (1.5 μM, an inhibitor of JAK3), or no inhibitor (Null). (e,f) Impact of NLRX1 depletion on viral replication in RIG-I-deficient Huh-7.5 cells or MAVS-deficient PH5CH8 cells. Immunoblots of NLRX1 in Huh-7.5 cells tranduced with NLRX1-T3 sgRNA (e), or NLRX1-MAVS double-deficient PH5CH8 cells (f) are shown on the left. GLuc analysis of HCV replication at 72 hpt or qRT-PCR of intracellular HAV RNA at 72 hpi are shown on the right. Each symbol (throughout) represents an individual technical replicate. Data shown are representative of 2-3 independent experiments, each with 2-3 technical replicates, and are shown mean ± S.E.M. ns = nonsignificant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 by two way ANOVA (a,c) or t test (b,d-f).
Supplementary Figure 2 NLRX1 deficiency minimally impairs NF-κB signaling in virus-infected PH5CH8 cells.
(a,b) Impact of NLRX1 depletion on SeV-triggered (a) IFNB 1-Luc and (b) IRF3-responsive (4*PRDI/III)-Luc promoter activation in PH5CH8 cells. (c) Measurement of IL6 mRNA decay in SeV-infected NLRX1-T3 cells. Cells were infected with SeV for 2.5 h prior to the addition of actinomycin D (Act D, 10 μg/ml). Data are presented as percent mRNA remaining relative to the starting abundance determined by qRT-PCR (set as 100%). Data were fit to a one-phase decay model using the least-squares method. (d) Dual luciferase reporter analysis of IL6-3′UTR-Luc in PH5CH8 cells with or without NLRX1 overexpression. EV, empty vector. (e) (left) Immunoblots of p-IκBα, IκBα, p-RELA (S536) and RELA in SeV-infected NLRX1-deficient PH5CH8 cells. (right) Quantitative analysis of p-IκBα and p-RELA from three independent experiments. (f) Immunoblot confirmation of IRF3 and IRF1 depletion in PH5CH8 cells. (g) qRT-PCR quantitation of IL1B and IL6 mRNA responses after SeV infection of IRF3- and IRF1-deficient PH5CH8 cells. (h) ELISA assay for IL-6 protein abundance in IRF1- and IRF3-deficient cells infected with SeV. Unless otherwise indicated, assays were performed 3 h after infection with SeV. Each symbol represents an individual technical replicate (a,b,d,g,h) or experiment (e). Data are representative of 3 (a,b,d), 5 (c) or 2 (g,h) independent experiments, each with 3 technical replicates. Data are presented as mean ± S.E.M. Two-way ANOVA (a,b,e,g,h) and t test (d) were used for statistical analysis. ns = nonsignificant, *p < 0.05, **p < 0.01, *** P < 0.001, ****P < 0.0001.
Supplementary Figure 3 SeV infection results in MAVS and NF-κB signaling that leads to an immediate IRF1 mRNA response in PH5CH8 cells.
(a) Immunoblots showing SeV-induced IRF3 dimerization and IRF1 expression in MAVS-deficient or NLRX1-MAVS double-deficient PH5CH8 cells. (b) Immunoblots showing SeV-triggered IRF1 protein responses with (Act D) or without (Null) actinomycin D pretreatment (10 μg/ml, 30 min) in NLRX1-T3 cells. (c-f) IRF1 protein and IRF1 mRNA responses in IRF3-deficient (c), RELA-deficient (d), NFKB1-depleted (siRNA) (e) and RELA-NFKB1 double-deficient (f) PH5CH8 cells. Each symbol (c,d,f) represents an individual technical replicate. Immunoblots are representative of 2-3 independent experiments. qRT-PCR results are representative of 2 experiments, each with 2-3 technical replicates, and are presented as mean ± S.E.M. ns = nonsignificant, **p < 0.01, ****P < 0.0001 by t test.
Supplementary Figure 4 NLRX1 deficiency globally impairs protein synthesis in virus-infected cells.
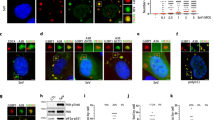
(a) qRT-PCR analysis of the impact of NLRX1 deficiency on SeV-induced IRF3 mRNA response in PH5CH8 cells. (b) Immunoblots showing IRF1 protein stability in SeV-infected control and NLRX1-T3 cells. Cells were infected with SeV for 3 h followed by inhibition of nascent protein synthesis with cyclohexamide (CHX, 100 μg/ml). See also Fig. 4c in the main text. (c) Immunoblots of IRF1 expression in SeV-infected NLRX1-T3 cells with (Puro) or without (Null) puromycin (50 μg/ml) pretreatment. (d) Phosphoimager analysis of SDS-PAGE of lysates of cells pulsed with [35S]-Met/Cys from 2.5-3.0 h following SeV or mock infection. See also Fig. 4d in the main text. (e) Puromycin incorporation assays demonstrating cellular protein synthesis in mock- or SeV-infected control versus NLRX1-T2 cells. Cells were pulse-labeled with puromycin (10 μg/ml) for 10 min prior to harvest at indicated timepoints. (top panels) Immunoblot showing puromycin-labelled nascent proteins with infrared fluorescence intensity traces of puromycin immunoblots at 0 (blue) or 3 h (red) after infection. (bottom) Confocal microscopic images of pulse-labeled NLRX1-T2 cells stained with antibody specific for puromycin. Scale bar, 40 μm. (f) Similar puromycin incorporation assays in primary HFHs with partial RNAi-mediated depletion of NLRX1. (g) Puromycin incorporation assay in primary N lrx1−/− versus wild-type MEFs following SeV infection. The far right panel shows normalized infrared intensity values of immunoblots from 4 independent experiments. (h) Representative A254 traces from mock- and SeV-infected control and NLRX1-T3 cells showing a sharp reduction in 80S translationally-competent ribosomes with an increase in 40S subunits in infected NLRX1-T3 cells. Each symbol represents an individual technical replicate (a) or experiment (g). Data are representative of 3 (a,c,e,f), 4 (g) or 6 (b,d) independent experiments, and are presented as mean ± S.E.M. ns = nonsignificant, ***p < 0.001 by two-way ANOVA (a) and *p < 0.5 by t test (g).
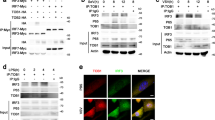
Supplementary Figure 5 NLRX1 protects virus-induced IRF1 responses from PKR–eIF2α-mediated global translational shutdown.
(a) Immunoblots of p-eIF2α in NLRX1-deficient PH5CH8 cells 8 h after transfection of poly(I:C) or HAV RNA. (b) (left) Global protein synthesis visualized by puromycin incorporation in PKR-deficient and PKR-NLRX1 double-deficient PH5CH8 cells following SeV infection. Scale bar, 40 μm. (right) Quantitative analysis of 3 independent experiments, with an average of 110 cells evaluated for each condition. *p< 0.05 by two-way ANOVA. (c) Immunoprecipitation was performed to ascertain whether there is a physical association between endogenous NLRX1 and PKR. Ctrl, isotype control antibody. (d) Similar immunoprecipitation assay following overexpression of NLRX1 and PKR in NLRX1-T3 cells. Data are representative of 3 (a-c) and 2 (d) experiments. (e) Schematic showing why NLRX1 is required for optimal early innate immune responses to RNA virus infections in hepatocytes. RNA virus infection initiates RIG-I/MAVS-dependent signaling, leading to early (3 h) dimerization of constitutively expressed IRF3 and NF-κB-mediated IRF1 transcription. In addition to acting as a previously identified brake on MAVS/IRF3 signaling, NLRX1 dampens PKR-mediated translational shutdown by competing with PKR for binding to viral RNA, thereby preventing inhibition of IRF1 protein synthesis. Since IRF1 is dominant over IRF3 in controlling the early cytokine response to virus infection in hepatocytes, NLRX1 depletion results in reduced cytokine transcription.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1 and 2. (PDF 1207 kb)
Rights and permissions
About this article
Cite this article
Feng, H., Lenarcic, E., Yamane, D. et al. NLRX1 promotes immediate IRF1-directed antiviral responses by limiting dsRNA-activated translational inhibition mediated by PKR. Nat Immunol 18, 1299–1309 (2017). https://doi.org/10.1038/ni.3853
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3853
This article is cited by
-
The NLR gene family: from discovery to present day
Nature Reviews Immunology (2023)
-
LncRNA MRF drives the regulatory function on monocyte recruitment and polarization through HNRNPD-MCP1 axis in mesenchymal stem cells
Journal of Biomedical Science (2022)
-
Impact of intracellular innate immune receptors on immunometabolism
Cellular & Molecular Immunology (2022)
-
mRNA vaccine: a potential therapeutic strategy
Molecular Cancer (2021)
-
Inflammasome activation and regulation: toward a better understanding of complex mechanisms
Cell Discovery (2020)