Abstract
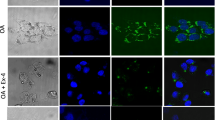
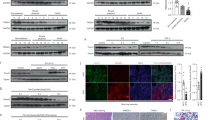
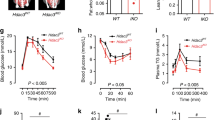
Nonalcoholic fatty liver disease is becoming the most common chronic liver disease in Western countries, and limited therapeutic options are available. Here we uncovered a role for intestinal hypoxia-inducible factor (HIF) in hepatic steatosis. Human-intestine biopsies from individuals with or without obesity revealed that intestinal HIF-2α signaling was positively correlated with body-mass index and hepatic toxicity. The causality of this correlation was verified in mice with an intestine-specific disruption of Hif2a, in which high-fat-diet-induced hepatic steatosis and obesity were substantially lower as compared to control mice. PT2385, a HIF-2α-specific inhibitor, had preventive and therapeutic effects on metabolic disorders that were dependent on intestine HIF-2α. Intestine HIF-2α inhibition markedly reduced intestine and serum ceramide levels. Mechanistically, intestine HIF-2α regulates ceramide metabolism mainly from the salvage pathway, by positively regulating the expression of Neu3, the gene encoding neuraminidase 3. These results suggest that intestinal HIF-2α could be a viable target for hepatic steatosis therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ray, K. NAFLD-the next global epidemic. Nat. Rev. Gastroenterol. Hepatol. 10, 621 (2013).
Chalasani, N. et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 142, 1592–1609 (2012).
Rotman, Y. & Sanyal, A.J. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut 66, 180–190 (2017).
Ju, C., Colgan, S.P. & Eltzschig, H.K. Hypoxia-inducible factors as molecular targets for liver diseases. J. Mol. Med. (Berl.) 94, 613–627 (2016).
Semenza, G.L. & Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 12, 5447–5454 (1992).
Ivan, M. et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Minamishima, Y.A. et al. A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol. Cell. Biol. 29, 5729–5741 (2009).
Qu, A. et al. Hypoxia-inducible transcription factor 2α promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology 54, 472–483 (2011).
Ramakrishnan, S.K. et al. HIF2α is an essential molecular brake for postprandial hepatic glucagon response independent of insulin signaling. Cell Metab. 23, 505–516 (2016).
Taniguchi, C.M. et al. Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nat. Med. 19, 1325–1330 (2013).
Wei, K. et al. A liver Hif-2α-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition. Nat. Med. 19, 1331–1337 (2013).
Gonzalez, F.J., Jiang, C. & Patterson, A.D. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 151, 845–859 (2016).
Singh, V. et al. Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell Metab. 22, 983–996 (2015).
Jiang, C. et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest. 125, 386–402 (2015).
Safran, M. et al. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc. Natl. Acad. Sci. USA 103, 105–110 (2006).
Pagadala, M., Kasumov, T., McCullough, A.J., Zein, N.N. & Kirwan, J.P. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol. Metab. 23, 365–371 (2012).
Kitatani, K., Idkowiak-Baldys, J. & Hannun, Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 20, 1010–1018 (2008).
Jornayvaz, F.R. & Shulman, G.I. Diacylglycerol activation of protein kinase Cɛ and hepatic insulin resistance. Cell Metab. 15, 574–584 (2012).
Xue, X. et al. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 145, 831–841 (2013).
Wallace, E.M. et al. A small-molecule antagonist of HIF2α is efficacious in preclinical models of renal cell carcinoma. Cancer Res. 76, 5491–5500 (2016).
Zou, Y., Albohy, A., Sandbhor, M. & Cairo, C.W. Inhibition of human neuraminidase 3 (NEU3) by C9-triazole derivatives of 2,3-didehydro-N-acetyl-neuraminic acid. Bioorg. Med. Chem. Lett. 20, 7529–7533 (2010).
Yoshinaga, A. et al. NEU3 inhibitory effect of naringin suppresses cancer cell growth by attenuation of EGFR signaling through GM3 ganglioside accumulation. Eur. J. Pharmacol. 782, 21–29 (2016).
Triner, D. & Shah, Y.M. Hypoxia-inducible factors: a central link between inflammation and cancer. J. Clin. Invest. 126, 3689–3698 (2016).
Kelly, C.J. et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17, 662–671 (2015).
Rivera-Chávez, F. et al. Depletion of butyrate-producing clostridia from the gut microbiota drives an aerobic luminal expansion of salmonella. Cell Host Microbe 19, 443–454 (2016).
Hubbard, T.D., Murray, I.A. & Perdew, G.H. Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab. Dispos. 43, 1522–1535 (2015).
Kawano, Y. et al. Colonic pro-inflammatory macrophages cause insulin resistance in an intestinal Ccl2/Ccr2-dependent manner. Cell Metab. 24, 295–310 (2016).
Rahman, K. et al. Loss of junctional adhesion molecule A promotes severe steatohepatitis in mice on a diet high in saturated fat, fructose, and cholesterol. Gastroenterology 151, 733–746 (2016).
Ramakrishnan, S.K. & Shah, Y.M. Role of intestinal HIF-2α in health and disease. Annu. Rev. Physiol. 78, 301–325 (2016).
Glover, L.E., Lee, J.S. & Colgan, S.P. Oxygen metabolism and barrier regulation in the intestinal mucosa. J. Clin. Invest. 126, 3680–3688 (2016).
Furuta, G.T. et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 193, 1027–1034 (2001).
Synnestvedt, K. et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 110, 993–1002 (2002).
Robinson, A. et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155 (2008).
Karhausen, J. et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 114, 1098–1106 (2004).
Glover, L.E. et al. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc. Natl. Acad. Sci. USA 110, 19820–19825 (2013).
Xie, L. et al. Hypoxia-inducible factor/MAZ-dependent induction of caveolin-1 regulates colon permeability through suppression of occludin, leading to hypoxia-induced inflammation. Mol. Cell. Biol. 34, 3013–3023 (2014).
Cummins, E.P. et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134, 156–165 (2008).
Shah, Y.M. et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology 134, 2036–2048.e3 (2008).
Summers, S.A. & Goodpaster, B.H. CrossTalk proposal: Intramyocellular ceramide accumulation does modulate insulin resistance. J. Physiol. (Lond.) 594, 3167–3170 (2016).
Chavez, J.A. & Summers, S.A. A ceramide-centric view of insulin resistance. Cell Metab. 15, 585–594 (2012).
Gorden, D.L. et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J. Lipid Res. 56, 722–736 (2015).
Haus, J.M. et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58, 337–343 (2009).
Chaurasia, B. & Summers, S.A. Ceramides—lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 26, 538–550 (2015).
Xia, J.Y. et al. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 22, 266–278 (2015).
Turpin, S.M. et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 (2014).
Raichur, S. et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20, 687–695 (2014).
Holland, W.L. et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 (2007).
Chaurasia, B. et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 24, 820–834 (2016).
Jiang, C. et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 6, 10166 (2015).
Perry, R.J., Samuel, V.T., Petersen, K.F. & Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510, 84–91 (2014).
Samuel, V.T. & Shulman, G.I. Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871 (2012).
Giussani, P., Tringali, C., Riboni, L., Viani, P. & Venerando, B. Sphingolipids: key regulators of apoptosis and pivotal players in cancer drug resistance. Int. J. Mol. Sci. 15, 4356–4392 (2014).
Yoshizumi, S. et al. Increased hepatic expression of ganglioside-specific sialidase, NEU3, improves insulin sensitivity and glucose tolerance in mice. Metabolism 56, 420–429 (2007).
Scaringi, R. et al. NEU3 sialidase is activated under hypoxia and protects skeletal muscle cells from apoptosis through the activation of the epidermal growth factor receptor signaling pathway and the hypoxia-inducible factor (HIF)-1α. J. Biol. Chem. 288, 3153–3162 (2013).
Cho, H. et al. On-target efficacy of a HIF2α antagonist in preclinical kidney cancer models. Nature 539, 107–111 (2016).
Chen, W. et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016).
Haase, V.H., Glickman, J.N., Socolovsky, M. & Jaenisch, R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc. Natl. Acad. Sci. USA 98, 1583–1588 (2001).
Anderson, E.R. et al. The hypoxia-inducible factor-C/EBPα axis controls ethanol-mediated hepcidin repression. Mol. Cell. Biol. 32, 4068–4077 (2012).
Hu, C.J., Sataur, A., Wang, L., Chen, H. & Simon, M.C. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1α and HIF-2α. Mol. Biol. Cell 18, 4528–4542 (2007).
Acknowledgements
We thank L.G. Byrd, Y. Zhang, X. Gao, W. Liu, X. Gong, and T. Yan (National Cancer Institute) for assistance with the mouse studies, and B. Liu and X. Wu (Chinese Academy of Sciences) for help with the immunohistochemistry. This project was funded in part by the National Cancer Institute Intramural Research Program to F.J.G., grants from the National Key Research and Development Program of China (2016YFC0903100, 2016YFC0903102) to Changtao Jiang, the National Natural Science Foundation of China (31401011 and 81522007 to CT. J., and 81403007 to X.C.), National Institutes of Health grants ES022186 to A.D.P., and CA148828 and DK095201 to Y.M.S. S.K.R. was supported by NIDDK (K99DK110537). Q.W. was supported by the Peak Talent Foundation of Jiangsu Province Hospital of Chinese Medicine (Y2014RC18) and Jiangsu Government Scholarship for Overseas Studies. D.S. and J.Z. were supported by fellowships from the Chinese Scholarship Council.
Author information
Authors and Affiliations
Contributions
C.X., T.Y., Y.L., X.L., T.C., Q.W., D.S., J.Z., S.K.R., L.S., Chunmei Jiang, X.X., Y.T., and K.W.K. performed the research and analyzed the data. C.X., A.D.P., Y.M.S., Y.W., Changtao Jiang and F.J.G. designed and supervised the research. C.X., Changtao Jiang and F.J.G. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures and Tables
Supplementary Figures 1–12 and Supplementary Tables 1–3 (PDF 5390 kb)
Rights and permissions
About this article
Cite this article
Xie, C., Yagai, T., Luo, Y. et al. Activation of intestinal hypoxia-inducible factor 2α during obesity contributes to hepatic steatosis. Nat Med 23, 1298–1308 (2017). https://doi.org/10.1038/nm.4412
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4412
This article is cited by
-
Associations between HIFs and tumor immune checkpoints: mechanism and therapy
Discover Oncology (2024)
-
HIF2α, Hepcidin and their crosstalk as tumour-promoting signalling
British Journal of Cancer (2023)
-
Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
Hexavalent Chromium Induces Cartilage Degeneration and Osteoarthritis Pathogenesis
Exposure and Health (2023)
-
SOX71, A Biocompatible Succinyl Derivative of the Triarylmethyl Radical OX071 for In Vivo Quantitative Oxygen Mapping Using Electron Paramagnetic Resonance
Molecular Imaging and Biology (2023)