Abstract
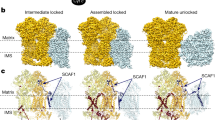
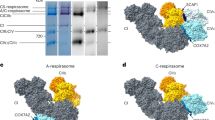
The oxidative phosphorylation electron transport chain (OXPHOS-ETC) of the inner mitochondrial membrane is composed of five large protein complexes, named CI–CV. These complexes convert energy from the food we eat into ATP, a small molecule used to power a multitude of essential reactions throughout the cell. OXPHOS-ETC complexes are organized into supercomplexes (SCs) of defined stoichiometry: CI forms a supercomplex with CIII2 and CIV (SC I+III2+IV, known as the respirasome), as well as with CIII2 alone (SC I+III2). CIII2 forms a supercomplex with CIV (SC III2+IV) and CV forms dimers (CV2). Recent cryo-EM studies have revealed the structures of SC I+III2+IV and SC I+III2. Furthermore, recent work has shed light on the assembly and function of the SCs. Here we review and compare these recent studies and discuss how they have advanced our understanding of mitochondrial electron transport.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nicholls, D.G. & Ferguson, S.J. Bioenergetics 4 (Academic Press, London, 2013).
Park, R.B. & Biggins, J. Quantasome: size and composition. Science 144, 1009–1011 (1964).
Enríquez, J.A. Supramolecular organization of respiratory complexes. Annu. Rev. Physiol. 78, 533–561 (2016).
Chance, B., Estabrook, R.W. & Lee, C.P. Electron transport in the oxysome. Science 140, 379–380 (1963).
Hatefi, Y., Haavik, A.G. & Griffiths, D.E. Studies on the electron transfer system. XL. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. J. Biol. Chem. 237, 1676–1680 (1962).
Chazotte, B. & Hackenbrock, C.R. The multicollisional, obstructed, long-range diffusional nature of mitochondrial electron transport. J. Biol. Chem. 263, 14359–14367 (1988).
Hackenbrock, C.R., Chazotte, B. & Gupte, S.S. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J. Bioenerg. Biomembr. 18, 331–368 (1986).
Schägger, H. & Pfeiffer, K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 19, 1777–1783 (2000).
Schägger, H. & Pfeiffer, K. The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J. Biol. Chem. 276, 37861–37867 (2001).
Iwata, S. et al. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc 1 complex. Science 281, 64–71 (1998).
Tsukihara, T. et al. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science 272, 1136–1144 (1996).
Davies, K.M. et al. Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc. Natl. Acad. Sci. USA 108, 14121–14126 (2011).
Allegretti, M. et al. Horizontal membrane-intrinsic α-helices in the stator a-subunit of an F-type ATP synthase. Nature 521, 237–240 (2015).
Hahn, A. et al. Structure of a complete atp synthase dimer reveals the molecular basis of inner mitochondrial membrane morphology. Mol. Cell 63, 445–456 (2016).
Greggio, C. et al. Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle. Cell Metab. 25, 301–311 (2017).
Barrientos, A. & Ugalde, C. I function, therefore I am: overcoming skepticism about mitochondrial supercomplexes. Cell Metab. 18, 147–149 (2013).
Acín-Pérez, R., Fernández-Silva, P., Peleato, M.L., Pérez-Martos, A. & Enríquez, J.A. Respiratory active mitochondrial supercomplexes. Mol. Cell 32, 529–539 (2008).
Shinzawa-Itoh, K. et al. Purification of active respiratory supercomplex from bovine heart mitochondria enables functional studies. J. Biol. Chem. 291, 4178–4184 (2016).
Acín-Pérez, R. et al. Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol. Cell 13, 805–815 (2004).
Moreno-Lastres, D. et al. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab. 15, 324–335 (2012).
Diaz, F., Fukui, H., Garcia, S. & Moraes, C.T. Cytochrome c oxidase is required for the assembly/stability of respiratory complex I in mouse fibroblasts. Mol. Cell. Biol. 26, 4872–4881 (2006).
Diaz, F., Enríquez, J.A. & Moraes, C.T. Cells lacking Rieske iron-sulfur protein have a reactive oxygen species-associated decrease in respiratory complexes I and IV. Mol. Cell. Biol. 32, 415–429 (2012).
Maranzana, E., Barbero, G., Falasca, A.I., Lenaz, G. & Genova, M.L. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid. Redox Signal. 19, 1469–1480 (2013).
Lopez-Fabuel, I. et al. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc. Natl. Acad. Sci. USA 113, 13063–13068 (2016).
Blaza, J.N., Serreli, R., Jones, A.J.Y., Mohammed, K. & Hirst, J. Kinetic evidence against partitioning of the ubiquinone pool and the catalytic relevance of respiratory-chain supercomplexes. Proc. Natl. Acad. Sci. USA 111, 15735–15740 (2014).
Lenaz, G., Tioli, G., Falasca, A.I. & Genova, M.L. Complex I function in mitochondrial supercomplexes. Biochim. Biophys. Acta 1857, 991–1000 (2016).
Bianchi, C., Genova, M.L., Parenti Castelli, G. & Lenaz, G. The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J. Biol. Chem. 279, 36562–36569 (2004).
Lapuente-Brun, E. et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340, 1567–1570 (2013).
Chen, Y.-C. et al. Identification of a protein mediating respiratory supercomplex stability. Cell Metab. 15, 348–360 (2012).
Ikeda, K., Shiba, S., Horie-Inoue, K., Shimokata, K. & Inoue, S. A stabilizing factor for mitochondrial respiratory supercomplex assembly regulates energy metabolism in muscle. Nat. Commun. 4, 2147 (2013).
Mourier, A., Matic, S., Ruzzenente, B., Larsson, N.-G. & Milenkovic, D. The respiratory chain supercomplex organization is independent of COX7a2l isoforms. Cell Metab. 20, 1069–1075 (2014).
Cogliati, S. et al. Mechanism of super-assembly of respiratory complexes III and IV. Nature 539, 579–582 (2016).
Pérez-Pérez, R. et al. COX7A2L is a mitochondrial complex III binding protein that stabilizes the III2+IV supercomplex without affecting respirasome formation. Cell Rep. 16, 2387–2398 (2016).
Letts, J.A., Fiedorczuk, K. & Sazanov, L.A. The architecture of respiratory supercomplexes. Nature 537, 644–648 (2016).
Gu, J. et al. The architecture of the mammalian respirasome. Nature 537, 639–643 (2016).
Sousa, J.S., Mills, D.J., Vonck, J. & Kühlbrandt, W. Functional asymmetry and electron flow in the bovine respirasome. eLife 5, e21290 (2016).
Wu, M., Gu, J., Guo, R., Huang, Y. & Yang, M. Structure of mammalian respiratory supercomplex I1III2IV1 . Cell 167, 1598–1609 e10 (2016).
Althoff, T., Mills, D.J., Popot, J.L. & Kühlbrandt, W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1 . EMBO J. 30, 4652–4664 (2011).
Dudkina, N.V., Kudryashev, M., Stahlberg, H. & Boekema, E.J. Interaction of complexes I, III, and IV within the bovine respirasome by single particle cryoelectron tomography. Proc. Natl. Acad. Sci. USA 108, 15196–15200 (2011).
Fiedorczuk, K. et al. Atomic structure of the entire mammalian mitochondrial complex I. Nature 538, 406–410 (2016).
Zhu, J., Vinothkumar, K.R. & Hirst, J. Structure of mammalian respiratory complex I. Nature 536, 354–358 (2016).
Gao, X. et al. Structural basis for the quinone reduction in the bc1 complex: a comparative analysis of crystal structures of mitochondrial cytochrome bc1 with bound substrate and inhibitors at the Qi site. Biochemistry 42, 9067–9080 (2003).
Yano, N. et al. The Mg2+-containing water cluster of mammalian cytochrome c oxidase collects four pumping proton equivalents in each catalytic cycle. J. Biol. Chem. 291, 23882–23894 (2016).
Sarewicz, M. & Osyczka, A. Electronic connection between the quinone and cytochrome C redox pools and its role in regulation of mitochondrial electron transport and redox signaling. Physiol. Rev. 95, 219–243 (2015).
Darrouzet, E., Cooley, J.W. & Daldal, F. The cytochrome bc (1) complex and its homologue the b (6) f complex: similarities and differences. Photosynth. Res. 79, 25–44 (2004).
Mitchell, P. The protonmotive Q cycle: a general formulation. FEBS Lett. 59, 137–139 (1975).
Milenkovic, D., Blaza, J.N., Larsson, N.-G. & Hirst, J. The enigma of the respiratory chain supercomplex. Cell Metab. 25, 765–776 (2017).
Stroud, D.A. et al. Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 538, 123–126 (2016).
Guerrero-Castillo, S. et al. The assembly pathway of mitochondrial respiratory chain complex i. Cell Metab. 25, 128–139 (2017).
Calvaruso, M.A. et al. Mitochondrial complex III stabilizes complex I in the absence of NDUFS4 to provide partial activity. Hum. Mol. Genet. 21, 115–120 (2012).
Davoudi, M., Kotarsky, H., Hansson, E., Kallijärvi, J. & Fellman, V. COX7A2L/SCAFI and pre-complex III modify respiratory chain supercomplex formation in different mouse strains with a Bcs1l mutation. PLoS One 11, e0168774 (2016).
Sterky, F.H. et al. Altered dopamine metabolism and increased vulnerability to MPTP in mice with partial deficiency of mitochondrial complex I in dopamine neurons. Hum. Mol. Genet. 21, 1078–1089 (2012).
Milenkovic, D. et al. TWINKLE is an essential mitochondrial helicase required for synthesis of nascent D-loop strands and complete mtDNA replication. Hum. Mol. Genet. 22, 1983–1993 (2013).
Hatle, K.M. et al. MCJ/DnaJC15, an endogenous mitochondrial repressor of the respiratory chain that controls metabolic alterations. Mol. Cell. Biol. 33, 2302–2314 (2013).
Williams, E.G. et al. Systems proteomics of liver mitochondria function. Science 352, aad0189 (2016).
Jha, P., Wang, X. & Auwerx, J. Analysis of mitochondrial respiratory chain supercomplexes using blue native polyacrylamide gel electrophoresis (BN-PAGE). Curr. Protoc. Mouse Biol. 6, 1–14 (2016).
Yanamura, W., Zhang, Y.Z., Takamiya, S. & Capaldi, R.A. Tissue-specific differences between heart and liver cytochrome c oxidase. Biochemistry 27, 4909–4914 (1988).
Van Kuilenburg, A.B., Van Beeumen, J.J. & Muijsers, A.O. Subunits VIIa, b,c of human cytochrome c oxidase. Eur. J. Biochem. 203, 193–199 (1992).
Hüttemann, M., Kadenbach, B. & Grossman, L.I. Mammalian subunit IV isoforms of cytochrome c oxidase. Gene 267, 111–123 (2001).
Hüttemann, M. et al. Mice deleted for heart-type cytochrome c oxidase subunit 7a1 develop dilated cardiomyopathy. Mitochondrion 12, 294–304 (2012).
Sun, F. et al. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell 121, 1043–1057 (2005).
Stroh, A. et al. Assembly of respiratory complexes I, III, and IV into NADH oxidase supercomplex stabilizes complex I in Paracoccus denitrificans. J. Biol. Chem. 279, 5000–5007 (2004).
Yip, C.-Y., Harbour, M.E., Jayawardena, K., Fearnley, I.M. & Sazanov, L.A. Evolution of respiratory complex I: “supernumerary” subunits are present in the alpha-proteobacterial enzyme. J. Biol. Chem. 286, 5023–5033 (2011).
Schägger, H. Respiratory chain supercomplexes of mitochondria and bacteria. Biochim. Biophys. Acta 1555, 154–159 (2002).
Andrews, B., Carroll, J., Ding, S., Fearnley, I.M. & Walker, J.E. Assembly factors for the membrane arm of human complex I. Proc. Natl. Acad. Sci. USA 110, 18934–18939 (2013).
Mileykovskaya, E. & Dowhan, W. Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem. Phys. Lipids 179, 42–48 (2014).
Scheres, S.H.W. Processing of structurally heterogeneous Cryo-EM data in RELION. Methods Enzymol. 579, 125–157 (2016).
Kotlyar, A.B. & Vinogradov, A.D. Slow active/inactive transition of the mitochondrial NADH-ubiquinone reductase. Biochim. Biophys. Acta 1019, 151–158 (1990).
Vinogradov, A.D. Catalytic properties of the mitochondrial NADH-ubiquinone oxidoreductase (complex I) and the pseudo-reversible active/inactive enzyme transition. Biochim. Biophys. Acta 1364, 169–185 (1998).
Babot, M. et al. ND3, ND1 and 39kDa subunits are more exposed in the de-active form of bovine mitochondrial complex I. Biochim. Biophys. Acta 1837, 929–939 (2014).
Zickermann, V. et al. Structural biology. Mechanistic insight from the crystal structure of mitochondrial complex I. Science 347, 44–49 (2015).
Sazanov, L.A. A giant molecular proton pump: structure and mechanism of respiratory complex I. Nat. Rev. Mol. Cell Biol. 16, 375–388 (2015).
Guarás, A. et al. The CoQH2/CoQ ratio serves as a sensor of respiratory chain efficiency. Cell Rep. 15, 197–209 (2016).
Ge, J. et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 527, 64–69 (2015).
Muller, F.L., Liu, Y. & Van Remmen, H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 279, 49064–49073 (2004).
Kussmaul, L. & Hirst, J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. USA 103, 7607–7612 (2006).
Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 (2009).
Pryde, K.R. & Hirst, J. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J. Biol. Chem. 286, 18056–18065 (2011).
Letts, J.A., Degliesposti, G., Fiedorczuk, K., Skehel, M. & Sazanov, L.A. Purification of ovine respiratory complex i results in a highly active and stable preparation. J. Biol. Chem. 291, 24657–24675 (2016).
Forman, H.J. & Azzi, A. On the virtual existence of superoxide anions in mitochondria: thoughts regarding its role in pathophysiology. FASEB J. 11, 374–375 (1997).
Forman, H.J. & Kennedy, J.A. Role of superoxide radical in mitochondrial dehydrogenase reactions. Biochem. Biophys. Res. Commun. 60, 1044–1050 (1974).
Boveris, A. & Chance, B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 134, 707–716 (1973).
Swierczek, M. et al. An electronic bus bar lies in the core of cytochrome bc1. Science 329, 451–454 (2010).
Crofts, A.R. et al. The Q-cycle reviewed: how well does a monomeric mechanism of the bc1 complex account for the function of a dimeric complex? Biochim. Biophys. Acta 1777, 1001–1019 (2008).
Mitchell, P. Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 56, 1–6 (1975).
Osyczka, A., Moser, C.C. & Dutton, P.L. Fixing the Q cycle. Trends Biochem. Sci. 30, 176–182 (2005).
de Vries, S., Albracht, S.P., Berden, J.A. & Slater, E.C. A new species of bound ubisemiquinone anion in QH2: cytochrome c oxidoreductase. J. Biol. Chem. 256, 11996–11998 (1981).
Quinlan, C.L., Gerencser, A.A., Treberg, J.R. & Brand, M.D. The mechanism of superoxide production by the antimycin-inhibited mitochondrial Q-cycle. J. Biol. Chem. 286, 31361–31372 (2011).
Ohnishi, T. & Trumpower, B.L. Differential effects of antimycin on ubisemiquinone bound in different environments in isolated succinate. cytochrome c reductase complex. J. Biol. Chem. 255, 3278–3284 (1980).
Dikanov, S.A. et al. Hydrogen bonds between nitrogen donors and the semiquinone in the Qi-site of the bc 1 complex. J. Biol. Chem. 282, 25831–25841 (2007).
Yu, C.A., Nagaoka, S., Yu, L. & King, T.E. Evidence for the existence of a ubiquinone protein and its radical in the cytochromes b and c1 region in the mitochondrial electron transport chain. Biochem. Biophys. Res. Commun. 82, 1070–1078 (1978).
Yu, C.A., Nagoaka, S., Yu, L. & King, T.E. Evidence of ubisemiquinone radicals in electron transfer at the cytochromes b and c 1 region of the cardiac respiratory chain. Arch. Biochem. Biophys. 204, 59–70 (1980).
de Vries, S., Berden, J.A. & Slater, E.C. Properties of a semiquinone anion located in the QH2:cytochrome c oxidoreductase segment of the mitochondrial respiratory chain. FEBS Lett. 122, 143–148 (1980).
Raha, S., McEachern, G.E., Myint, A.T. & Robinson, B.H. Superoxides from mitochondrial complex III: the role of manganese superoxide dismutase. Free Radic. Biol. Med. 29, 170–180 (2000).
Xia, D. et al. Structural analysis of cytochrome bc1 complexes: implications to the mechanism of function. Biochim. Biophys. Acta. 1827, 1278–1294 (2013).
Genova, M.L. & Lenaz, G. Functional role of mitochondrial respiratory supercomplexes. Biochim. Biophys. Acta. 1837, 427–443 (2014).
Gupte, S. et al. Relationship between lateral diffusion, collision frequency, and electron transfer of mitochondrial inner membrane oxidation-reduction components. Proc. Natl. Acad. Sci. USA 81, 2606–2610 (1984).
Kröger, A. & Klingenberg, M. The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur. J. Biochem. 34, 358–368 (1973).
Kröger, A. & Klingenberg, M. Further evidence for the pool function of ubiquinone as derived from the inhibition of the electron transport by antimycin. Eur. J. Biochem. 39, 313–323 (1973).
Cruciat, C.M., Brunner, S., Baumann, F., Neupert, W. & Stuart, R.A. The cytochrome bc1 and cytochrome c oxidase complexes associate to form a single supracomplex in yeast mitochondria. J. Biol. Chem. 275, 18093–18098 (2000).
Mileykovskaya, E. et al. Arrangement of the respiratory chain complexes in Saccharomyces cerevisiae supercomplex III2IV2 revealed by single particle cryo-electron microscopy. J. Biol. Chem. 287, 23095–23103 (2012).
Trouillard, M., Meunier, B. & Rappaport, F. Questioning the functional relevance of mitochondrial supercomplexes by time-resolved analysis of the respiratory chain. Proc. Natl. Acad. Sci. USA 108, E1027–E1034 (2011).
Schneider, H., Lemasters, J.J., Höchli, M. & Hackenbrock, C.R. Fusion of liposomes with mitochondrial inner membranes. Proc. Natl. Acad. Sci. USA 77, 442–446 (1980).
Schneider, H., Lemasters, J.J., Höchli, M. & Hackenbrock, C.R. Liposome-mitochondrial inner membrane fusion. Lateral diffusion of integral electron transfer components. J. Biol. Chem. 255, 3748–3756 (1980).
Strogolova, V., Furness, A., Robb-McGrath, M., Garlich, J. & Stuart, R.A. Rcf1 and Rcf2, members of the hypoxia-induced gene 1 protein family, are critical components of the mitochondrial cytochrome bc1-cytochrome c oxidase supercomplex. Mol. Cell. Biol. 32, 1363–1373 (2012).
Vukotic, M. et al. Rcf1 mediates cytochrome oxidase assembly and respirasome formation, revealing heterogeneity of the enzyme complex. Cell Metab. 15, 336–347 (2012).
Zhou, A. et al. Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. eLife 4, e10180 (2015).
Acknowledgements
This work was supported in part by European Union's 2020 Research and Innovation Program under grant 701309.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Letts, J., Sazanov, L. Clarifying the supercomplex: the higher-order organization of the mitochondrial electron transport chain. Nat Struct Mol Biol 24, 800–808 (2017). https://doi.org/10.1038/nsmb.3460
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3460
This article is cited by
-
SCAF1 drives the compositional diversity of mammalian respirasomes
Nature Structural & Molecular Biology (2024)
-
ALKBH5 facilitates CYP1B1 mRNA degradation via m6A demethylation to alleviate MSC senescence and osteoarthritis progression
Experimental & Molecular Medicine (2023)
-
A concerted ATPase cycle of the protein transporter AAA-ATPase Bcs1
Nature Communications (2023)
-
Super-complex supercomplex
Nature Plants (2023)
-
Succinate dehydrogenase is essential for epigenetic and metabolic homeostasis in hearts
Basic Research in Cardiology (2023)