Abstract
Background
Propranolol is the first-choice treatment for severe infantile hemangioma (IH). However, 10– 30% of lesions relapse after propranolol treatment. The mechanisms underlying IH recurrence after propranolol treatment have not been completely elucidated.
Methods
This study combined an examination of hemodynamic changes with research regarding hemangioma stem cells (hscs) with differentially expressed microRNAs (miRNAs) to identify the factors affecting IH recurrence after propranolol treatment. Hemodynamic changes were monitored in 21 recurrent cases using high-frequency color Doppler ultrasound, and hscs were treated with different concentrations of propranolol. The levels of differentially expressed miRNAs and the activity of related pathways were then compared between 18 recurrent and 20 non-recurrent IH cases.
Results
During treatment, lesion depth and vessel density decreased, and the lesion resistance index increased. Obvious lesions and vessel signals were observed in recurrent cases compared with non-recurrent cases. Propranolol effectively inhibited hscs proliferation. Twenty-two differentially expressed miRNAs were found in the recurrent group compared with the non-recurrent group.
Conclusion
Recurrence may be attributed to a combination of events. Serum biomarkers and drug treatments for IH recurrence must be studied further.
Similar content being viewed by others
Main
Infantile hemangioma (IH) is the most common benign tumor in infants. Although most hemangiomas regress spontaneously and thus do not require treatment; ~10–20% of hemangiomas require urgent treatment during their proliferative phase (1). Because of its high efficacy and minimal adverse reactions, propranolol has been the first-line treatment for rapidly proliferating hemangiomas since 2008 (refs 2, 3, 4). In our previous large prospective trial, most patients achieved good therapeutic results; however, 92 of 679 patients experienced recurrence, with a recurrence rate of 13.5% (ref. 5). The mechanisms underlying this phenomenon have not yet been elucidated. The present study combined an evaluation of hemodynamic changes with research regarding hemangioma stem cells (hscs) with microRNAs (miRNAs) that were differentially expressed between recurrent and non-recurrent patients to preliminarily identify the factors affecting IH recurrence after propranolol treatment.
Methods
Patients and Therapy
The Declaration of Helsinki protocols were followed, and the procedures were approved by the ethics committee of Shanghai Ninth People’s Hospital (200806). The methods were conducted in accordance with the relevant guidelines, and informed consent was obtained from all subjects’ parents before samples of each subject’s serum were collected. From December 2008 to May 2014, 1,015 IHs were indicated for propranolol treatment after being assessed by two independent plastic surgeons, in accordance with the inclusion and exclusion criteria (6). Propranolol was administered to patients at a dosage of 2 mg/kg/d (in two divided doses separated by an interval of 12 h; that was slowly increased over the first 3 days of treatment). Propranolol was gradually stopped over a period of 2 weeks, either when patients exceeded 12 months of age or when no lesions remained (7). Among the patients enrolled in this study, 679 finished therapy and completed the subsequent follow-up period (3–17 months). Patients who had received therapies before receiving propranolol were excluded from the present study to manage outcome expectations.
Monitoring of Hemodynamic Changes by Color Doppler Ultrasound
A total of 21 recurrent patients who had undergone detailed color Doppler ultrasound (Mylab Touch; Esaote S.p.A., Genoa, Italy) assessments before propranolol treatment and 1 and 3 months after treatment were included in this study. Lesion depths, vessel densities, and lesion resistance indices (RIs) were recorded and analyzed using Student’s t-test (8). The significance threshold was set to 0.05.
Proliferation of Hscs After Propranolol Treatment
Hscs were isolated from clinically resected specimens, as reported by Khan et al. (9). Briefly, hemangioma samples were minced into small pieces using a scalpel, digested using collagenase (Roche, Indianapolis, IN), and filtered through a 100-μm cell strainer. Cells expressing CD133 were selected from this single-cell suspension using a magnetic microbead cell-sorting system (Miltenyi Biotec, Bergisch Gladbach, Germany) and plated on fibronectin-coated plates in endothelial cell growth media (EGM-2; Lonza, Basel, Switzerland) supplemented with 20% fetal bovine serum (Invitrogen, Carlsbad, CA), penicillin, and streptomycin (Invitrogen). Cells from passages 5 to 10 were used for all experiments. A total of 2.0 QUOTE 103 hscs were seeded in each well of a 96-well plate containing EGM-2 with or without 90 or 150 μM propranolol. The hscs were counted after 24, 48, and 72 h of culture. The medium was changed to EBM-2 after 12, 24, or 48 h of culture. The hscs were counted after 24, 48, and 72 h of culture. Cell numbers were assessed using Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan) and an Absorbance Microplate Reader (Biotek, ELx800, Winooski, USA).
Differential miRNA Expression in Recurrent Cases
Serum samples were collected from 18 recurrent and 20 non-recurrent patients before propranolol treatment. Inclusion criteria were the volume of a hemangioma greater than 5 cm3, and age 5 months or younger to ensure that data were captured while hemangiomas were still in the proliferation stage. Potential biomarkers were compared between the two groups using next-generation sequencing (10), and differentially expressed miRNAs were analyzed using Student’s t-test. The significance threshold was set to 0.05. To predict the genes targeted by the differentially expressed miRNAs, two computational target prediction algorithms (TargetScan 50 and miRanda 3.3a) were used to identify miRNA-binding sites. The data predicted by both algorithms were combined, and the overlaps were calculated. In addition, the Gene ontology (GO) terms and Kyoto encyclopedia genes and genomes (KEGG) pathways of these differentially expressed miRNA targets were annotated, and enrichment analyses based specifically on GO and KEGG were performed.
Results
Propranolol Treatment Outcomes
Ninety-two of the 679 patients enrolled in this study experienced recurrence. The female:male ratio in this group of patients was 2.7:1. Eight recurrent infants were born prematurely, and six had low birth weights (<2,500 g). The IHs were deep in 38 cases and mixed in 54 cases, and the mean age at presentation was 11 days (range: 0–42 days). The hemangiomas generally enlarged between 3.5 and 13 weeks after birth. The mean age at propranolol treatment initiation was 3.5 months (range: 1–7.8 months) and the mean age at propranolol treatment termination was 7.8 months (range: 3–17 months).
Monitoring of Hemodynamic Changes by Color Doppler Ultrasound
Compared with their pretreatment values, the mean lesion depth and vessel density decreased significantly during treatment, and the lesion RI increased significantly during treatment. Obvious lesions, abundant blood signals, and relatively low RIs were observed after recurrence, and residual lesions were observed in 19 cases when propranolol was tapered. However, no obvious lesions were observed in the other two cases in which propranolol was tapered. Figure 1 and Table 1 present the changes in depth, vessel density, and the RI that occurred in the recurrent group during the course of treatment.
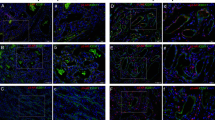
A typical recurrent case. (a) Before treatment, a 4-month-old boy presented with a large mixed hemangioma in his the right parotid region. (b) After 1 month of propranolol treatment, marked regression of both the superficial and the deep components of the hemangioma was observed. (c) Seven months later, no obvious lesion remained, and propranolol was stopped. (d,h,l) One month later, progressive regrowth of the deep component of the hemangioma was observed. (e–g) A decrease in the lesion depth from before treatment to 1 and 7 months after treatment. (i–k) A decrease in lesion vessel density from before treatment to 1 and 7 months after treatment. (m–o) An increase in the lesion RI from before treatment to 1 and 7 months after treatment. (p) A relatively low RI was observed after recurrence. RI, resistance index.
Hscs Proliferation After Propranolol Treatment
Propranolol exerted an antiproliferative effect on hscs, whose proliferation decreased with increasing propranolol concentrations. In particular, after 48 and 72 h of culture, the hscs in the 90 and 150 μM propranolol groups exhibited significantly lower proliferative activity than the blank group. Overall, proliferation increased after propranolol was removed. A significant increase in proliferation occurred when propranolol was removed at earlier stages (Figure 2).
Hscs proliferation with or without propranolol disposing. (a) A total of 2.0 QUOTE 103 hscs were seeded into each well of a 96-well plate in EGM-2 with or without 90 or 150 μM propranolol. The hscs were counted after 24, 48, and 72 h of culture. In particular, after 48 and 72 h of culture, the hscs in the 90 and 150 μM propranolol groups exhibited evidently lower proliferative activity than the hscs in the blank group. The medium was changed to EBM-2 after 12 (b), 24 (c), or 48 h (d) of culture. Overall, proliferation increased after propranolol was removed. A significant increase in proliferation occurred when propranolol was removed at earlier stages. EGM, endothelial cell growth media; Hscs, hemangioma stem cell; RI, resistance index.
Differential miRNA Expression in Recurrent Cases
We detected miRNA expression levels in serum samples collected from 18 recurrent and 20 non-recurrent patients before propranolol treatment. Genome-wide expression profiling of serum miRNAs based on normalized deep-sequencing counts demonstrated that the serum samples from the recurrent group contained 14 upregulated miRNAs and 8 downregulated miRNAs compared with the serum samples from the non-recurrent group (Table 2). We identified multiple miRNAs that were differentially expressed (P<0.05, fold change >1.5) between the recurrent and non-recurrent groups, including six miRNAs that were underexpressed in the recurrent group (hsa-miR-122-3p, hsa-miR-122-5p, mmu-miR-370-3p, hsa-miR-483-3p, hsa-miR-758-3p, and hsa-miR-494-3p) and two miRNAs that were overexpressed in the recurrent group (aca-let-7b-5p and hsa-let-7c-5p).
Putative Targets for miRNAs
A total of 17,259 target genes were obtained by predicting the genes targeted by the above-mentioned 22 differentially expressed miRNAs. Several potential targets for hsa-miR-122-3p, hsa-miR-122-5p, mmu-miR-370-3p, hsa-miR-483-3p, hsa-miR-758-3p, has-miR-494-3p, aca-let-7b-5p, and has-let-7c-5p were predicted using an online software (Table 3). These targets were mainly related to vascular remodeling, cell growth and differentiation, cell signaling, and cell adhesion. Given the hypothesized pathogenesis of IHs, we performed enrichment analysis based on GO and KEGG, the results of which indicated that cell adhesion molecules, MAPK signaling, focal adhesion, and phosphatidylinositol signaling pathways may have a close relationship with IH recurrence (Figure 3).
Discussion
The results of previous reports indicated that a 10–30% of IHs recur rate after termination of propranolol treatment (11, 12). The recurrence rate in our previous study (5) was 13.5% (92/679). There is no clear definition of recurrence. In the present study, IH recurrence was defined as the presence of obvious primary lesion regrowth and abundant blood flow, as detected by color Doppler ultrasound. Because they did not require further treatment, lesions with slight regrowth and without persistent growth were excluded from this study. As most recurrent IHs require a long treatment course, they inevitably impose intense psychological and economic burdens on patients’ families.
To date, the effects of propranolol on proliferative IHs have been attributed to vasoconstriction, angiogenesis inhibition, and apoptosis induction (13). However, direct evidence supporting this hypothesis is scarce, and the mechanism underlying IH recurrence after propranolol treatment remains poorly understood. Therefore, research regarding IH recurrence after propranolol treatment may not only facilitate elucidation of the mechanisms underlying the effects of propranolol treatment on IHs but also improve the clinical effectiveness of propranolol treatment and reduce IH recurrence rates. In the present study, we aimed to explore the factors causing IH recurrence after propranolol treatment by combining studies of hemodynamic changes with research regarding hscs with differentially expressed miRNAs.
Given its potential side effects, particularly its effects on the central nervous system (14, 15), propranolol is rarely used until IHs are completely involuted. Our patients were required to stop treatment when no obvious lesions were observed via color Doppler ultrasound monitoring or when the patients exceeded 12 months of age. However, complete involution was rarely observed, as residual lesions were observed in most cases. In our previous prospective study, we determined that recurrent cases were more common among patients whose lesions were difficult to resolve in a subsequent treatment course than among patients whose lesions were not difficult to treat (5). Among our 21 recurrent cases that underwent systematic color Doppler monitoring during treatment, residual lesions were observed in 19 cases when propranolol was tapered. Therefore, recurrent lesions may originate from residual lesions. Accelerating residual lesion involution may have a key role in improving propranolol clinical efficacy and lowering IH recurrence rates.
In our previous study, we found that large numbers of proliferating endothelial cells remained in recurrent cases (16). Proliferating IHs comprise endothelial cells, hscs, and pericytes, among other cells. Hscs are undoubtedly the most important of these cells (17). In the study described herein, hscs were treated with different concentrations of propranolol. We demonstrated that propranolol inhibited hsc proliferation more significantly as its concentration increased and that cell proliferation increased when propranolol was removed. Proliferation also increased more significantly when propranolol was removed at earlier stages than when the drug was removed at later stages. Therefore, residual hscs in lesions that were difficult to treat exhibited increased proliferative activity after propranolol was stopped. Wong et al. assumed that hscs continue to proliferate, albeit at a slow rate, which may explain the rebound growth that occurs after propranolol therapy has been tapered (18). In summary, we believe that residual lesions are an important cause of recurrence.
In 2008, Léauté-Labrèze et al. first described the change in the color of an IH that occurs within 24 h of treatment initiation and attributed the color change to the rapid onset of the effects of propranolol treatment. This author also observed lesion-softening (19). We frequently observed marked fading of the cutaneous color of lesions within a short time after propranolol therapy initiation and observed accelerated vessel regression after weeks or months of therapy. These findings suggest that propranolol exerts its effects by first inducing IH vasoconstriction and then decreasing IH vessel density. IH vessels are composed of endothelial cells and pericytes. Blood flow control is initiated in the capillaries, and pericytes likely regulate blood flow at the capillary level. The perivascular locations and morphology of hemangioma-derived pericytes suggest that they are contractile cells involved in regulating capillary blood flow in response to vasoactive agents (20). Lee et al. found that propranolol acted on proliferating IHs by increasing pericytic contractility (21). Because of a lack of sophisticated instruments, there is no direct evidence indicating whether propranolol can directly induce vasoconstriction in lesions. In our study, during treatment, lesion vessel density decreased, and the lesion RI increased, indirectly indicating that vessels in IH lesions constrict after propranolol treatment. In contrast, after recurrence, lesion vessel density increased, and the lesion RI decreased, which may be caused by residual vessel relaxation after propranolol treatment. Thus, vessel constriction in IH lesions after propranolol treatment may be an essential mechanism underlying the effects of propranolol treatment on IHs.
Vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, MMP-9, interleukin-6, and other growth factors are associated with IH progression (22, 23). Propranolol reduces the expression of these growth factors and inhibits angiogenesis (24). By monitoring IHs, we observed that lesion vessel density decreased during treatment, indicating that anti-angiogenesis is likely to be another mechanism by which propranolol affects IHs.
The molecular pathogenesis underlying IH recurrence is not well understood. Advances in genetic and epigenetic analyses have provided researchers with extremely valuable approaches for elucidating disease mechanisms, as well as approaches with the potential to identify biomarkers and/or pathways, thereby contributing to the development of better IH treatments, including molecularly targeted IH therapies. To the best of our knowledge, there are no representative serum biomarkers of IHs, which may be due to the difficulty of collecting serum samples from IH patients, as well as from normal infants. We have long been interested in studying serum biomarkers and found that vascular endothelial growth factor and angiogenin may be useful biomarkers of IHs (22, 25). In the present study, we had hoped to find specific biomarkers in the serum samples of recurrent cases that would facilitate the identification of recurrent cases before propranolol treatment. Oral corticosteroids, local injections, or surgery may be alternatives to propranolol in these patients. Further studies regarding related pathways may facilitate the discovery of new drug treatments for recurrent patients.
MiRNAs have been demonstrated to have essential roles in the pathogenesis of several human diseases. These molecules also have key roles in gene expression regulation (26). Moreover, miRNAs are known to participate in several important biological processes, such as embryonic development, differentiation, apoptosis, cell proliferation, and oncogenesis (27, 28). They are also relatively stable in blood. The formation of vascular tumors, including IH, is partly related to abnormal and excessive angiogenesis, and miRNAs can regulate the responses of vascular endothelial cells to angiogenic stimuli (29). It was reported that miR-126 is a positive regulator of angiogenic signaling and vascular endothelial integrity and that other miRNAs, such as miR-27b and miR-let-7f, exert pro-angiogenic effects, as their expression promotes angiogenesis (30). A recent study (31) focusing on miRNA has revealed that C19MC miRNAs were solely found in IH specimens other than normal tissues or other vascular anomalies. In this study, they showed that C19MC miRNAs were synthesized and secreted only by glucose transporter 1-positive IH endothelial cells, suggesting a potential role of C19MC miRNAs as an IH-specific biomarker. In addition, circulating C19MC miRNAs level was found to be correlated with IH size, proliferating rate, and resistance to propranolol treatment, which may become a useful tool for accurate prognosis. However, there are no studies regarding global miRNA expression in IHs or miRNA expression in recurrent IHs after propranolol treatment. To the best of our knowledge, the only published study regarding miRNAs in hemangiomas determined that miR-424 is underexpressed in senile hemangioma and that this miRNA may regulate abnormal proliferation (32). Therefore, we intended to identify differentially expressed miRNAs in recurrent cases that may represent useful biomarkers that can be used to improve diagnosis, prognosis, and treatment. Several studies have reported that the miRNAs expressed during mesenchymal stem cell (MSC) differentiation lack specificity because of variations in MSCs among different individual donors (33). Thus, it is difficult to identify specific miRNAs in MSCs and their differentiated cells based on information from a few donors. To more efficiently and accurately identify specific serum biomarkers of recurrence, we selected a large number of serum samples, namely, 18 recurrent and 20 non-recurrent serum samples, for next-generation sequencing after propranolol treatment. The two groups were similar with respect to lesion proliferation rates, volumes, locations, and types before propranolol treatment. They were also similar with respect to age and gender. Twenty-two miRNAs were differentially expressed between the recurrent and non-recurrent groups. On the basis of our results pertaining to fold changes in the expression of specific miRNAs, we selected five underexpressed miRNAs (hsa-miR-122-3p, hsa-miR-122-5p, hsa-miR-483-3p, hsa-miR-758-3p, and hsa-miR-494-3p) and one overexpressed miRNA (hsa-let-7c-5p) from the recurrent group as potential biomarkers. Several of these miRNAs have been identified as regulators and biomarkers in cancers. A recent study also reported that the let-7 family of miRNAs was potential biomarkers for grade prediction in breast cancer (34). MiR-483 has an important role in suppressing the deleted in liver cancer-1 (DLC-1) gene and may thus serve as a new therapeutic target and a potential biomarker of colorectal cancer (35), and miR-494 has been demonstrated to inhibit ovarian cancer cell proliferation and promote apoptosis by targeting fibroblast growth factor receptor 2 (ref. 36).
Predicting the target genes of miRNAs represents a basis for further experimental research on miRNA-regulatory mechanisms. We predicted the putative target genes of the above candidate miRNAs using TargetScan and Miranda and obtained several potential targets, according to the results of a meta-analysis that identified 54 deregulated genes involved in IH and angiogenesis. These genes are related to processes such as vascular remodeling, cell growth and differentiation, cell signaling, cell adhesion, and basement membrane/metabolism (37). Angiopoietin2 in the Tie2/Angiopoietin signaling pathway is linked to the pathogenesis of IH (38).
Enrichment analyses based on GO and KEGG demonstrated that cell adhesion molecules, as well as the MAPK signaling, focal adhesion, and phosphatidylinositol signaling pathways, may be closely related to IH recurrence. As the current study was limited by its relatively small number of recurrent cases and the laborious task of serum collection, verification of serum biomarkers and related pathways will be conducted in our future lines of work.
Recurrence is rarely observed in the natural IH regression process. A comparative study regarding recurrent cases and patients exhibiting natural regression may better elucidate the mechanisms underlying recurrence. Differentially expressed miRNAs and their dynamic changes during treatment can also be a focus of future research. Future studies on related pathways will help support the development of new drug treatments for recurrent cases.
Conclusions
Propranolol may exert its effects by evoking pericyte-mediated vasoconstriction and then decreasing IH vessel density. Propranolol can also effectively inhibit hscs proliferation. The antihemangioma effects of propranolol are probably not associated with a single mechanism but rather with a combination of mechanisms. In the present study, most of the recurrent cases were partial regressions, indicating that recurrent lesions may originate from residual lesions. Biomarkers were differentially expressed between recurrent and non-recurrent cases. Although certain IHs relapse after propranolol treatment, propranolol should remain the first-line treatment for proliferating IHs, as recurrent cases may be cured after a second propranolol treatment. However, additional drugs are needed to overcome the shortcomings of propranolol.
References
Haggstrom AN, Drolet BA, Baselga E et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics 2006;118:882–7.
Izadpanah A, Izadpanah A, Kanevsky J, Belzile E, Schwarz K . Propranolol versus corticosteroids in the treatment of infantile hemangioma: a systematic review and meta-analysis. Plast Reconstr Surg 2013;131:601–13.
Darrow DH, Greene AK, Mancini AJ et al. Diagnosis and management of infantile hemangioma. Pediatrics 2015;136:e1060–104.
Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med 2015;372:735–46.
Chang L, Ye X, Qiu Y et al. Is propranolol safe and effective for outpatient use for infantile hemangioma? A prospective study of 679 cases from one center in China. Ann Plast Surg 2016;76:559–63.
Szychta P, Stewart K, Anderson W . Treatment of infantile hemangiomas with propranolol: clinical guidelines. Plast Reconstr Surg 2014;133:852–62.
Siegfried EC, Keenan WJ, Al-Jureidini S . More on propranolol for hemangiomas of infancy. N Engl J Med 2008;359:2846–7.
Kutz AM, Aranibar L, Lobos N, Wortsman X . Color Doppler ultrasound follow-up of infantile hemangiomas and peripheral vascularity in patients treated with propranolol. Pediatr Dermatol 2015;32:468–75.
Khan ZA, Boscolo E, Picard A et al. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest 2008;118:2592–9.
South AP, Li Q, Uitto J . Next-generation sequencing for mutation detection in heritable diseases: the paradigm of pseudoxanthoma elasticum. J Invest Dermatol 2015;135:937–40.
Shehata N, Powell J, Dubois J et al. Late rebound of infantile hemangioma after cessation of oral propranolol. Pediatr Dermatol 2013;30:587–91.
Saqi L, Zvulunov A, Lapidoth M, Ben Amitai D . Efficacy and safety of propranolol for the treatment of infantile hemangioma: a presentation of ninety-nine cases. Dermatology 2014;228:136–44.
Ji Y, Chen S, Xu C, Li L, Xiang B . The use of propranolol in the treatment of infantile haemangiomas: an update on potential mechanisms of action. Br J Dermatol 2015;172:24–32.
Tozzi A . Oral propranolol for infantile hemangioma. N Engl J Med 2015;373:284.
Moyakine AV, Hermans DJ, Fuijkschot J, van der Vleuten CJ . Propranolol treatment of infantile hemangiomas does not negatively affect psychomotor development. J Am Acad Dermatol 2015;73:341–2.
Chang L, Ma G, Jin Y et al. Recurrence of infantile hemangioma after termination of propranolol treatment. Ann Plast Surg 2014;72:173–5.
Thuy L, Marcelo H . Pathogenesis of infantile hemangioma. Facial Plast Surg 2012;28:554–62.
Wong A, Hardy KL, Kitajewski AM, Shawber CJ, Kitajewski JW, Wu JK . Propranolol accelerates adipogenesis in hemangioma stem cells and causes apoptosis of hemangioma endothelial cells. Plast Reconstr Surg 2012;130:1012–21.
Léauté-Labrèze C, Dumas de laRoque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A . Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649–51.
Hamilton NB, Attwell D, Hall CN . Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics 2010;2 pii 5.
Lee D, Boscolo E, Durham JT, Mulliken JB, Herman IM, Bischoff J . Propranolol targets the contractility of infantile haemangioma-derived pericytes. Br J Dermatol 2014;171:1129–37.
Zhang L, Lin X, Wang W et al. Circulating level of vascular endothelial growth factor in differentiating hemangioma from vascular malformation patients. Plast Reconstr Surg 2005;116:200–4.
Greenberger S, Bischoff J . Pathogenesis of infantile haemangioma. Br J Dermatol 2013;169:12–9.
Zhang L, Mai HM, Zheng J et al. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol 2013;7:48–55.
Jiang C, Lin X, Hu X et al. Angiogenin: a potential serum marker of infantile hemangioma revealed by cDNA microarray analysis. Plast Reconstr Surg 2014;134:231e–9e.
Chen X, Ba Y, Ma L et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008;18:997–1006.
Bartel DP . MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97.
Cummins JM, Velculescu VE . Implications of micro-RNA profiling for cancer diagnosis. Oncogene 2006;25:6220–7.
Boscolo E, Bischoff J . Vasculogenesis in infantile hemangioma. Angiogenesis 2009;12:197–207.
Fish JE, Srivastava D . MicroRNAs: opening a new vein in angiogenesis. Sci Signal 2009;2:pe1.
Strub GM, Kirsh AL, Whipple ME et al. Endothelial and circulating C19MC microRNAs are biomarkers of infantile hemangioma. JCI Insight 2016;1:e88856.
Nakashima T, Jinnin M, Etoh T et al. Down-regulation of mir-424 contributes to the abnormal angiogenesis via MEK1 and cyclin E1 in senile hemangioma: its implications to therapy. PLoS ONE 2010;5:e14334.
Goff LA, Boucher S, Ricupero CL et al. Differentiating human multipotent mesenchymal stromal cells regulate microRNAs: prediction of microRNA regulation by PDGF during osteogenesis. Exp Hematol 2008;36:1354–69.
Oztemur Y, Bekmez T, Aydos A, Yulug IG, Bozkurt B, Dedeoglu BG . A ranking-based meta-analysis reveals let-7 family as a meta-signature for grade classification in breast cancer. PLoS ONE 2015;10:e0126837.
Cui H, Liu Y, Jiang J et al. IGF2-derived miR-483 mediated oncofunction by suppressing DLC-1 and associated with colorectal cancer. Oncotarget 2016; 7:48456-66.
Zhao X, Zhou Y, Chen YU, Yu F . miR-494 inhibits ovarian cancer cell proliferation and promotes apoptosis by targeting FGFR2. Oncol Lett 2016;11:4245–51.
Bertoni N, Pereira LM, Severino FE, Moura R, Yoshida WB, Reis PP . Integrative meta-analysis identifies microRNA-regulated networks in infantile hemangioma. BMC Med Genet 2016;17:4.
Ji Y, Chen S, Li K, Li L, Xu C, Xiang B . Signaling pathways in the development of infantile hemangioma. J Hematol Oncol 2014;7:13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Statement of Financial Support
This work was supported by grants of the National Natural Science Foundation of China (81272127, 81601699) and the Joint Research Project on Important Disease of Shanghai Health System (2013ZYJB0014).
Rights and permissions
About this article
Cite this article
Chang, L., Lv, D., Yu, Z. et al. Infantile hemangioma: factors causing recurrence after propranolol treatment. Pediatr Res 83, 175–182 (2018). https://doi.org/10.1038/pr.2017.220
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.220