Abstract
Background
The Neonatal Intensive Care Unit Network Neurobehavioral Scale (NNNS) is a standardized method for infant neurobehavioral assessment. Normative values are available for newborns, but the NNNS is not always feasible at birth. Unfortunately, 1-month NNNS normative data are lacking.
Aims
To provide normative data for the NNNS examination at 1 month and to assess birth-to-one-month changes in NNNS summary scores.
Study design
The NNNS was administered at birth and at 1 month within a longitudinal prospective study design.
Subjects
A cohort of 99 clinically healthy full-term infants were recruited from a well-child nursery.
Outcome measures
Birth-to-1-month NNNS variations were evaluated and the association of neonatal and sociodemographic variables with the rate of change of NNNS summary scores were investigated.
Results and conclusions
NNNS scores from the 10th to the 90th percentile represent a range of normative performance at 1 month. A complex pattern of stability and change emerged comparing NNNS summary scores from birth to 1 month. Orienting, Regulation, and Quality of movements significantly increased, whereas Lethargy and Hypotonicity significantly decreased. Birth-to-1-month changes in NNNS performance suggest improvements in neurobehavioral organization. These data are useful for research purposes and for clinical evaluation of neurobehavioral performance in both healthy and at-risk 1-month-old infants.
Similar content being viewed by others
Main
The Neonatal Intensive Care Unit Network Neurobehavioral Scale (NNNS) (1, 2, 3) is a standardized method for infant neurobehavioral assessment. It is a valid biomarker for early detection of developmental delay in at-risk populations. Previous studies highlighted the efficacy of the NNNS in the early neurobehavioral screening of clinical (e.g., in utero drugs exposure (4), maternal depression (5), neonatal exposure to methadone or buprenorphine (6)), and at-risk infants (e.g., preterm birth (7)). Moreover, the NNNS assessment has been successfully used in prospective studies. For example, associations between early neurobehavioral assessment and short- and long-term outcomes have been documented, including behavioral outcomes (8) and psychomotor development (9).
Although NNNS extreme scores (too low and too high) are indicative of less-than-optimal development and risk conditions, the NNNS was not conceived within a neuropathological framework. Rather, it was framed by a developmental perspective, highlighting normative values for the neurobehavioral performance and its variations (1). Thus, its application to a healthy population is needed in order to provide a “broader and more nuanced view of the neurobehavior of the typical newborn” (10). From a clinical point of view, the establishment of normative data is crucial in order to detect less-than-optimal trajectories of neurobehavioral development. Additionally, studies on normative samples may identify subtle associations between medical or demographic variables and problems in neurobehavioral performance not previously appreciated (11).
Normative NNNS data in healthy newborns assessed from 24 to 28 h postpartum have been previously published (11). Moreover, the NNNS has been administered to a sample of healthy infants at different chronological ages within the first month of life (mean=2.2 weeks; range 0.3–4.8 weeks) providing descriptive data within the first weeks after birth (12). However, in this study infants’ age varied widely and longitudinal changes from birth to 1 month could not be traced because of a cross-sectional design, nor could normative values be obtained. Indeed, normative values at 1 month are still lacking. This is surprising since the first month of life represents a particularly vulnerable time for infants, characterized by critical developmental processes which lead to greater neurobehavioral organization (13). Moreover, the availability of 1-month NNNS normative values might be useful clinically, as the assessment of the neurobehavioral profile is generally delayed when infants are not clinically stable during the first days of life (3). Thus, 1-month NNNS normative values can support the daily clinical activity of neonatologists and pediatricians who work with healthy and at-risk infants.
The aims of the present study were (a) to provide normative values for the NNNS at 1 month in healthy infants; (b) to investigate changes in healthy infants’ NNNS neurobehavioral profile from birth to 1 month of life; and (c) to identify perinatal and sociodemographic factors associated with significant changes in NNNS summary scores.
Methods
The present study reports on a cohort of infants enrolled from the well-child nurseries of a Boston teaching hospital. One hundred infants were consecutively contacted from a previous cohort study on at-birth NNNS examination. Ninety-nine infants participated to the follow-up NNNS examination at 1 month. All infants had adequate birth weight for gestational age. Eligibility of the mother–infant dyads in the newborn period was determined from medical records and nursing reports. Inclusion criteria were well-newborn nursery stay and discharge from the hospital within 4 days, while exclusion criteria were circumcision within 12 h of examination, stay in the neonatal intensive care unit for more than 12 h, major physical or neurologic anomaly, human immunodeficiency virus positive, and positive toxicology screen for cocaine or heroin. Mothers without language barriers were recruited regardless of race, ethnicity, marital status, or education. Maternal exclusion criteria included major cognitive deficits, personality disorders, or psychosis. Infants and mothers were clinically healthy. Informed written consent was obtained from the mother. The Institutional Review/Ethical Board of the Brigham Women’s Hospital approved this research project. All the procedures have been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Neurobehavioral evaluation
The NNNS (3) is a 128-item standardized (14) assessment to evaluate the neurobehavioral status of healthy and high-risk infants. It has 13 summary scores: Habituation, Attention, Arousal, Regulation, Handling procedures, Quality of movement, Excitability, Lethargy, Nonoptimal reflexes, Asymmetric reflexes, Hypertonicity, Hypotonicity, and Stress/abstinence scale. The NNNS has good internal and concurrent validity (14).
Infants’ perinatal variables and clinical
Infants’ perinatal characteristics were abstracted from the medical records, and included birth weight, birth length, gestational age, Apgar at minute 1, Apgar at minute 5, duration of labor, type of delivery, and complications during the postpartum period (e.g., major brain lesions, neurosensorial deficits, syndromes, malformations). Total risk was assessed with the Hobel score (15) by trained medical personnel. Previous research have fixed a clinical risk cutoff for Hobel scores ≥10 (16).
Parents’ sociodemographic variables
Parents filled out a sociodemographic form. Data were collected on parents’ age (years), ethnicity (White/Caucasian, Black/African-American, Hispanic, Asian, other), mother’s and father’s work status (full-time, part-time, not working), income (<US$10,000, US $10,001–25,000, US$25,001–50,000, US$50,001–75,000, US $75,001–100,000, >US$100,000), insurance (Medicaid/other government insurance vs. HMO/private insurance) and marital status (cohabitant vs. single or separated parent). Family socioeconomic status (SES) using the Hollinghead (17) determined by the more prestigious occupational level of either parent. Scores ranged from 0 to 90. Lower scores reflect lower SES.
Procedures
Standard procedures for NNNS assessment at birth and at 1 month are fully described in the previous literature. (11, 12, 13, 14, 15, 16, 17, 18) Informed consent was obtained from parents for both examinations. To insure both the validity and reliability of examination and data, several exceptional procedures not typical of clinical research on the NNNS were put in place. At both time points, the NNNS was administered by two certified clinicians, who were blinded to neonatal status. Reliability was set to the criteria used in other studies (11): no more than two 2-point disagreements on items with 9-point scales. For 5-point scale items or less, agreement had to be exact. In total, no more than five disagreements for the complete assessment were accepted. To ensure a high level of reliability, every examination was observed and scored independently by a second examiner. Disagreements were resolved in conference. To further ensure reliability and administrative quality, random examinations were further scored by an NNNS trainer observing and then independently scoring the examination and evaluating agreement with the examiners. In addition, the 1-month NNNS assessment occurred in a follow-up visit in a light and temperature controlled child development laboratory.
Statistics and data reduction
Descriptive statistics for each summary score were calculated for the NNNS evaluations at birth and at 1 month of age. A multivariate analysis of variance with time of NNNS screening (2 levels: birth vs. 1 month) as the within-group factor was used on the NNNS summary scores to test for significant neurobehavioral variations from birth to 1 month of age. Separate univariate repeated-measure analyses of variance were then applied to each NNNS scale to assess their change from birth to 1 month. Pearson’s bivariate correlations were used to assess the rank-order stability of each NNNS summary score from birth to 1 month. To adjust for multiple comparisons, we used the Benjamini and Hochberg criterion, with q<0.05. Potential predictors of 1-month NNNS summary scores were checked for multicollinearity, using Pearson’s and Spearman’s coefficient for continuous and categorical predictors. If two or more variables were significantly correlated, only one was included in the final set of potential predictors according to clinically and theoretically relevant criteria. For each of the NNNS scores, the final set of potential predictors were regressed on the difference score computed by subtracting NNNS summary score at birth to NNNS summary score at 1 month (i.e., Δ scores). Predictors were entered or dropped in a stepwise manner in the regression models with Δ NNNS summary scores to evaluate change from birth to 1 month as dependent variables. All the analyses were conducted with SPSS 21.0, at P<0.05.
Results
Neonatal and demographic descriptors
Clinical and sociodemographic information for the sample are provided in Table 1. The values indicate that the infants and their mothers were at low social risk and were homogenous for neonatal, clinical, and sociodemographic variables.
One-month NNNS normative data
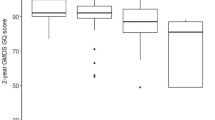
Descriptive data for the NNNS assessments at birth and at 1 month are provided in Table 2. Most of 1-month-old infants (80.8%) were not in the required sleep state for Habituation compared to 57.27% in the newborn period, so this scale was excluded from further analyses. Normative cutoffs for the remaining scales are provided in Figure 1. As in the previous NNNS normative data study (11), the mean, SD, range, and percentile values for each scale are provided.
Graphical representation of 1-month normative data. Note: The light gray area defines theoretical range for each NNNS summary scores. The box plots indicate normative data as follows: mean is the central tendency index; the box defines 10th and 90th percentile values; the whiskers extend to the observed minimum and maximum values for the present sample.
Stability and change in NNNS performance
An overall multivariate significant effect was detected, F(12,68)=3.07, P<0.00, η2p=0.35 (see Table 2). After adjusting with the Benjamini–Hochberg (1995) criterion, significant correlations emerged for Arousal (r=0.20, P<0.05) and Quality of movements (r=0.25, P<0.01). Birth-to-1-month significant correlations were also documented for Regulation (r=0.22, P<0.05) and Handling (r=0.21, P<0.05), but they did not survive the multiple comparison adjustment.
Neonatal factors associated with NNNS performance
Selected predictors were: birth weight (g), Apgar at minute 1, Hobel score, and family SES. The regression model was significant for Δ Quality of movement, R2=0.08, F(1,84)=6.96, P=0.01, and for Δ Hypotonicity, R2=0.06, F(1,84)=5.16, P=0.02. Lower Hobel score at birth was predictive of greater change in Quality of movement from birth to 1 month, B=−0.15, 95% confidence interval (CI): −2.27, −0.04, β=−0.28, t=−2.64, P=0.01. Higher birth weight at birth was predictive of greater reduction in Hypotonicity from birth to 1 month, B=0.00, 95% CI: 0.00, 0.00, β=0.24, t=2.27, P=0.02.
Discussion
This paper presents normative NNNS data for a sample of healthy infants assessed at birth and at 1 month of life. It provides a standard comparison to evaluate infants’ neurobehavior at 1 month of life. Consistent with Fink et al. (11) we considered the 10th and 90th percentiles as cutoff points for normative performance (see Figure 1). Scores exceeding these values at 1 month might indicate less-than-optimal development and the presence of subtle risk conditions.
Owing to the infants’ healthy status, it was not surprising that a very low and narrow range of NNNS summary scores emerged that were associated with neurobehavioral indexes of illness or developmental risk (i.e., Asymmetrical reflexes, Hypertonicity, Hypotonicity, Stress/Abstinence) (12). Similarly, low normative values emerged for Excitability and Lethargy. Nonetheless, few subjects (6.1% and 9.1%, respectively) manifested high scores (7-to-13 and 4-to-10, respectively) on these scales, suggesting the presence of little individual variability in healthy infants even at 1 month. Thus, an infant with high scores on these scales might be more closely monitored. By contrast, large individual variability was observed for Handling procedures and Nonoptimal reflexes. The findings suggest that the infants’ ability to be soothed and regulated after external stimulation, as well as the emergence of optimal reflexes is still ongoing (18). Moreover, these findings extend previous evidence (11), confirming NNNS sensitivity to depict individual differences in a healthy population. Mid-range normative scores were found for Regulation and Arousal, with limited variability in the sample. Notably, extreme scores (too low and too high) are indicative of nonoptimal development and risk conditions for the infants. As such, it is not surprising that mid-range normative scores were observed in the present healthy sample of 1-month-old infants. The highest scores were obtained for the Orienting summary score, with very few infants (8.2%) scoring below the median. Since more than 90% of the healthy infants were able to be alert and to shift attention in response to stimuli, it seems that scores below 5 may be a potential marker of neurodevelopmental risk at 1 month.
The second aim of the present study was to assess change of NNNS summary scores from birth to 1 month in healthy infants. Neurobehavioral development during the first month of life showed a mixed pattern of stability and variation. First, as regard to Habituation, due to the sleep-state-dependent nature of items, very limited data were available for the 1-month screening (19.2% at 1 month vs. 51.5% at birth). While previous studies reported similar percentages for birth-screening (54.4% [19] and 47% (11)), in the only published paper on 1-month-old healthy infants Habituation was excluded from the analyses (12). In sum, Habituation appears somewhat inapplicable in the healthy 1-month infants’ assessment. It appears that at this age infants’ organization of sleep and awake states is rapidly changing and only a small proportion of infants might be in the required state for the procedures of the Habituation summary score. However, the very inapplicability of habituation at 1 month compared to the newborn period may be a marker of significant neurobehavioral organization and maturation in the immediate postnatal period, that is, infants are more alert and aroused. Nonetheless, the habituation scale might be of use when the NNNS is applied to infants at risk for neurobehavioral development.
Second, seven NNNS summary scores did not change from birth to 1 month (i.e., Arousal, Handling procedures, Excitability, Nonoptimal reflexes, Asymmetrical reflexes, Hypertonicity, and Stress/abstinence). Additionally, rank-order correlations in the present study were at best moderate and they were significant for only a limited subset of summary scores. Nonchanging scores are plausibly related to the absence of clinical concerns in our sample, especially for nonoptimal reflexes, asymmetry, hyper-tone, and stress/abstinence. For the healthy sample included here, these scores were already low at birth and continued to be flat at 1 month. These summary scores are designed to evaluate neurologic functioning by the counting the number of nonoptimal signs, which are generally absent or very few in a sample of healthy newborns, but they nonetheless remain critical markers of developmental risk (11).
Five NNNS summary scores (i.e., Orienting, Regulation, Quality of movements, Lethargy, and Hypotonia) significantly changed from birth to 1 month. On the one hand, these significant changes appear to reflect a maturational shift in the neurobehavior of healthy infants during the first 4 weeks of life. They are consistent with previous literature on newborns behavioral and neurologic development (20, 21). For example, Hadders-Algra and Prechtl (20) observed infants’ motor and neurological development from 2 to 18 weeks, suggesting developmental trajectory from “writhing” to “swipes and swats” movements. On the other side, it should be noted that specific neonatal variables in the nonclinical range were nonetheless predictive of the change in scores for Quality of movement and Hypotonicity. Lower Hobel score at birth was predictive of greater increase in Quality of movement from birth to 1 month. As Fink et al. (11) documented an association between lower prenatal risk (Hobel score) and better quality of movement, these findings extend previous evidence further confirming NNNS sensitivity in the face of minimal or nonclinical risk conditions. Finally, lower birth weight was associated with greater reduction in Hypotonicity. This may be consistent with the fact that low birthweight newborns reported more Hypotonicity at the newborn NNNS assessment (11). As such, the present findings suggest the presence of a greater recovery of tonicity in healthy infants who had low scores at birth (22). It is also noteworthy that some variables, such as mode of delivery, were not related to 1-month performance. More importantly, the findings of relations between nonclinical levels of some variables (e.g., birthweight, risk scores) suggest the power of these variables to affect behavior and perhaps that cutoff levels for these variables may be misleading as to their effects on development.
There are limitations to this study. The limited nonclinical range of medical and demographic variables likely underestimates their relations to neurobehavioral performance, even if they add evidence about NNNS robustness and sensitivity. Moreover, the usefulness of the normative data provided in the present sample of healthy infants needs to be tested for clinical validity in the context of at risk or clinically ill 1-month-old infants. Certainly it would now be valuable to study a large and heterogeneous sample of infants. The strengths of the present work include the use of a prospective design, assessing NNNS in a longitudinal way from birth to 1 month of age. Moreover, the percentage of children who returned to the 1-month assessment was very high (i.e., 99%). Finally, the procedure strictly adhered to NNNS administration guidelines.
Conclusion
The present study provides standardized normative scores for the neurobehavioral examination of healthy infants at 1 month of age. To date, this is the first study presenting data on healthy infant neurobehavioral development in the first month of life in a longitudinal manner. The significant neurobehavioral changes from birth to 1 month clearly suggest that the normative values available for newborns (11) cannot be used to characterize the neurobehavior of 1-month olds. Although developmental changes of specific NNNS summary scores are likely related to maturational shifts and/or even subclinical neonatal variables, the availability of standardized percentiles reported in this paper appears to be a prospectively valid criterion to evaluate the neurobehavioral performance of healthy infants. Moreover, the present normative data should be considered as a criteria to evaluate the presence of specific abnormalities and deficits in the neurobehavioral profile of infants at different risk conditions in the very early postnatal life. Additionally, since pediatricians might face obstacles in assessing clinically at-risk newborns during the immediate hours after birth (3), whereas a visit at or around 1 month is typical in standard practice, the normative values provided here make for a reliable comparison criteria for typical infants and for infants with concerning clinical conditions.
References
Lester BM, Tronick EZ . History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics 2004;113 (3, Part 2): 634–640.
Lester BM, Tronick EZ, LaGasse L et al. Summary statistics of neonatal intensive care unit network neurobehavioral scale scores from the maternal lifestyle study: a quasinormative sample. Pediatrics 2004;113:668–675.
Lester BM, Tronick EZ, Brazelton TB . The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics 2004;113 (3): 641–667.
Lester BM, Tronick EZ, LaGasse L et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics 2002;110 (6): 1182–1192.
Salisbury AL, Lester BM, Seifer R et al. Prenatal cocaine use and maternal depression: effects on infant neurobehavior. Neurotoxicol Teratol. 2007;29 (3): 331–340.
Coyle MG, Salisbury AL, Lester BM et al. Neonatal neurobehavior effects following buprenorphine versus methadone exposure. Addiction 2012;107 (Suppl 1): 63–73.
Montirosso R, Del Prete A., Bellu R, Tronick E, Borgatti R, Group NAC for Q of L (NEO-AS. Level of NICU quality of developmental care and neurobehavioral performance in very preterm infants. Pediatrics 2012;129 (5): e1129–e1137.
Liu J, Bann C, Lester B et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics 2010;125 (1): e90–e98.
Sucharew H, Khoury JC, Xu Y, Succop P, Yolton K . NICU Network Neurobehavioral Scale Profiles Predict Developmental Outcomes in a LOW-RISK SAMPLE. Paediatr Perinat Epidemiol. 2012;26 (4): 344–352.
Tronick E, Lester BM . Grandchild of the NBAS: The NICU Network Neurobehavioral Scale (NNNS): a review of the research using the NNNS. J Child Adolesc Psychiatr Nurs 2013;26 (3): 193–203.
Fink NS, Tronick E, Olson K, Lester B . Healthy newborns’ neurobehavior: norms and relations to medical and demographic factors. J Pediatr. 2012;161 (6): 1073–1079.e3.
Spittle AJ, Walsh J, Olsen JE et al. Neurobehaviour and neurological development in the first month after birth for infants born between 32–42 weeks’ gestation. Early Hum Dev 2016;96:7–14.
Lester BM, Miller RJ, Hawes K et al. Infant neurobehavioral development. Semin Perinatol 2011;35 (1): 8–19.
Noble Y, Boyd R . Neonatal assessments for the preterm infant up to 4 months corrected age: a systematic review. Dev Med Child Neurol 2012;54 (2): 129–139.
Hobel CJ, Youkeles L, Forsythe A . Prenatal and intrapartum high-risk screening: II. Risk factors reassessed. Am J Obstet Gynecol 1979;135 (8): 1051–1056.
Maloni JA, Kane JH, Suen L, Wang KK . Dysphoria among high-risk pregnant hospitalized women on bed rest: a longitudinal study. Nurs Res 2002;51 (2): 92–99.
Hollingshead AB . Four Factor Index of Social Status. New Haven, CT: Yale University, 1968.
DeSantis A, Harkins D, Tronick E, Kaplan E, Beeghly M . Exploring an integrative model of infant behavior: What is the relationship among temperament, sensory processing, and neurobehavioral measures? Infant Behav Dev 2011;34 (2): 280–292.
Tronick EZ, Olson K, Rosenberg R, Bohne L, Lu J, Lester BM . Normative neurobehavioral performance of healthy infants on the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics 2004;113 (3, Part 2): 676–678.
Hadders-Algra M, Prechtl HF . Developmental course of general movements in early infancy. I. Descriptive analysis of change in form. Early Hum Dev 28 (3): 201–213.
Mirmiran M, Lunshof S . Perinatal development of human circadian rhythms. Prog Brain Res 1996;111:217–226.
Brown N, Spittle A . Neurobehavioral evaluation in the preterm and term infant. Curr Pediatr Rev 2014;10 (1): 65–72.
Acknowledgements
This study was supported by Standardization of the NRN-Neurobehavioral Scale, National Institute of Child Health and Human Development (R01Hd37138 to E.T.). The supporting agency has no role in study design, collection, analysis and interpretation of data, writing of the manuscript, and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Provenzi, L., Olson, K., Giusti, L. et al. NICU Network Neurobehavioral Scale: 1-month normative data and variation from birth to 1 month. Pediatr Res 83, 1104–1109 (2018). https://doi.org/10.1038/pr.2018.25
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2018.25
This article is cited by
-
Impaired in vivo feto-placental development is associated with neonatal neurobehavioral outcomes
Pediatric Research (2023)
-
Randomized clinical trial investigating the effect of consistent, developmentally-appropriate, and evidence-based multisensory exposures in the NICU
Journal of Perinatology (2021)
-
Development of an abbreviated symptom score for the neonatal abstinence syndrome
Journal of Perinatology (2020)
-
Psychosocial and medical adversity associated with neonatal neurobehavior in infants born before 30 weeks gestation
Pediatric Research (2020)