Abstract
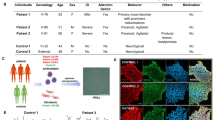
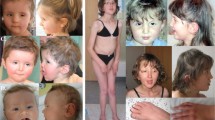
Bloom syndrome (BS) is a rare autosomal recessive disorder characterized by genomic instability that leads to various complications, including cancer. Given the low prevalence of BS in Japan, we conducted a nationwide survey. We recruited eight patients with BS, three of whom exhibited intellectual disability. The 631delCAA mutation in the BLM gene was detected in 9 out of 16 alleles. To investigate neuronal development in patients with BS, we generated induced pluripotent stem cells derived from one of these patients (BS-iPSCs). We examined the phenotypes of the induced cortical neurons derived from the generated BS-iPSCs using a previously reported protocol; the generated BS-iPSCs showed an approximately 10-times higher frequency of sister-chromatid exchange (SCE) than the control iPSCs. Immunocytochemistry revealed shorter axons and higher proliferative potential in BS-iPSC-derived cortical neurons compared with control iPSCs. To our knowledge, our study is the first to clarify the abnormality of the cortical neuron phenotypes derived from patients with BS. Our findings may help identify the pathogenesis of neuronal differentiation in BS and aid in the development of novel therapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, Ciocci S, et al. The Bloom’s syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655–66.
Chaganti RS, Schonberg S, German J. A manyfold increase in sister chromatid exchanges in Bloom’s syndrome lymphocytes. Proc Natl Acad Sci USA. 1974;71:4508–12.
McDaniel LD, Schultz RA. Elevated sister chromatid exchange phenotype of Bloom syndrome cells is complemented by human chromosome 15. Proc Natl Acad Sci USA. 1992;89:7968–72.
Raynard S, Bussen W, Sung P. A double Holliday junction dissolvasome comprising BLM, topoisomerase IIIα, and BLAP75. J Biol Chem. 2006;281:13861–4.
Yin J, Sobeck A, Xu C, Meetei AR, Hoatlin M, Li L, et al. BLAP75, an essential component of Bloom’s syndrome protein complexes that maintain genome integrity. EMBO J. 2005;24:1465i1476.
Singh TR, Ali AM, Busygina V, Raynard S, Fan Q, Du CH, et al. BLAP18/RMI2, a novel OB-fold-containing protein, is an essential component of the Bloom helicase-double Holliday junction dissolvasome. Genes Dev. 2008;22:2856–68.
Martin CA, Sarlós K, Logan CV, Parry DA, Bizard AH, Leitch A, et al. Mutations in TOP3A cause a Bloom syndrome-like disorder. Am J Hum Genet. 2018;103:221–31.
Hudson DF, Amor DJ, Boys A, Butler K, Williams L, Zhang T, et al. Loss of RMI2 increases genome instability and causes a Bloom-like syndrome. PLoS Genet. 2016;12:1–24.
Cunniff C, Bassetti JA, Ellis NA. Bloom’s syndrome: clinical spectrum, molecular pathogenesis, and cancer predisposition. Mol Syndromol. 2017;8:4–23.
Masmoudi A, Marrakchi S, Kamoun H, Chaaben H, Salah GB, Salah RB, et al. Clinical and laboratory findings in 8 patients with Bloom’s syndrome. J Dermatol Case Rep. 2012;6:29–33.
Classen CF, Riehmer V, Landwehr C, Kosfeld A, Heilmann S, Scholz C, et al. Dissecting the genotype in syndromic intellectual disability using whole exome sequencing in addition to genome-wide copy number analysis. Hum Genet. 2013;132:825–41.
Gönenc II, Wolff A, Schmidt J, Zibat A, Müller C, Cyganek L, et al. Single-cell transcription profiles in Bloom syndrome patients link BLM deficiency with altered condensin complex expression signatures. Hum Mol Genet. 2022;31:2185–93.
Luo G, Santoro IM, McDaniel LD, Nishijima I, Mills M, Youssoufian H, et al. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nat Genet. 2000;26:424–9.
Tereshchenko IV, Chen Y, McDaniel LD, Schultz RA, Tischfield JA, Shao C. Small scale genetic alterations contribute to increased mutability at the X-linked Hprt locus in vivo in Blm hypomorphic mice. DNA Repair (Amst). 2010;9:551–7.
Gatinois V, Desprat R, Becker F, Pichard L, Bernex F, Isidor B, et al. iPSC line derived from a Bloom syndrome patient retains an increased disease-specific sister-chromatid exchange activity. Stem Cell Res J. 2020;43:101696.
Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–12.
Ohuchi K, Funato M, Kato Z, Seki J, Kawase C, Tamai Y, et al. Established stem cell model of spinal muscular atrophy is applicable in the evaluation of the efficacy of thyrotropin-releasing hormone analog. Stem Cells Transl Med. 2016;5:152–63.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Kondo T, Asai M, Tsukita K, Kutoku Y, Ohsawa Y, Sunada Y, et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell. 2013;12:487–96.
Schoenaker MHD, Henriet SS, Zonderland J, van Deuren M, Pan-Hammarström Q, Posthumus-van Sluijs SJ, et al. Immunodeficiency in Bloom’s syndrome. J Clin Immunol. 2018;38:35–44.
Kaneko H, Isogai K, Fukao T, Matsui E, Kasahara K, Yachie A, et al. Relatively common mutations of the Bloom syndrome gene in the Japanese population. Int J Mol Med. 2004;14:439–42.
Oka Y, Hamada M, Nakazawa Y, Muramatsu H, Okuno Y, Higasa K, et al. Digenic mutations in ALDH2 and ADH5 impair formaldehyde clearance and cause a multisystem disorder, AMeD syndrome. Sci Adv. 2020;18:6.
Bouman A, van Koningsbruggen S, Karakullukcu MB, Schreuder WH, Lakeman P. Bloom syndrome does not always present with sun-sensitive facial erythema. Eur J Med Genet. 2018 ;61:94–7.
Bulfone A, Smiga SM, Shimamura K, Peterson A, Puelles L, Rubenstein JL. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 1995;15:63–78.
Bulfone A, Wang F, Hevner R, Anderson S, Cutforth T, Chen S, et al. An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron. 1998;21:1273–82.
Medina L, Legaz I, Gonzalez G, De Castro F, Rubenstein JL, Puelles L. Expression of Dbx1, Neurogenin 2, Semaphorin 5A, Cadherin 8, and Emx1 distinguish ventral and lateral pallial histogenetic divisions in the developing mouse claustroamygdaloid complex. J Comp Neurol. 2004;474:504–23.
Remedios R, Huilgol D, Saha B, Hari P, Bhatnagar L, Kowalczyk T, et al. A stream of cells migrating from the caudal telencephalon reveals a link between the amygdala and neocortex. Nat Neurosci. 2007;10:1141–50.
Hachiya Y, Motonaga K, Itoh M, Masuko T, Enomoto T, Sonobe H, et al. Immunohistochemical expression and pathogenesis of BLM in the human brain and visceral organs. Neuropathology. 2001;21:123–8.
Toba T, Nishimuta T, Funabashi S, Adachi M. Serum protein levels in healthy Japanese children, with special regard to 5 classes of immunoglobulin (author’s transl)]. Rinsho Byori. 1975;23:763–9.
Acknowledgements
The authors thank the patients who participated in this study as well as their families. We would also like to thank Dr. K. Osafune (Kyoto University) for providing the human iPSC line 201B7.
Funding
This study was supported in part by the Health and Labor Sciences Research Grants for Research on Measures for Intractable Diseases of the Ministry of Health, Labour and Welfare.
Author information
Authors and Affiliations
Contributions
Research design: MF, CK, YI, AY, and HK. Experiments, data acquisition, and data analysis: CK and HK. Manuscript writing: HK.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The generation and pathological analysis (including human gene analysis) of patient-derived iPSCs in this study were approved by the Ethical Review Committee of the National Hospital Organization, Nagara Medical Center (approval no. 26-15). Established human stem cells were handled according to revisions of the guidelines for clinical research using human stem cells by the Ministry of Health, Labour and Welfare of Japan. Written informed consent was obtained from the Bloom syndrome patient for the publication of this report. The 201B7 line was provided by Dr. K. Osafune (Kyoto University).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaneko, H., Kawase, C., Seki, J. et al. Intellectual disability and abnormal cortical neuron phenotypes in patients with Bloom syndrome. J Hum Genet 68, 321–327 (2023). https://doi.org/10.1038/s10038-023-01121-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-023-01121-9