Abstract
Background
Obesity is associated with impaired primary and secondary immune responses to influenza infection, with T cells playing a critical role. T-cell function is highly influenced by the cellular metabolic state; however, it remains unknown how altered systemic metabolism in obesity alters T-cell metabolism and function to influence immune response. Our objective was to identify the altered cellular metabolic state of T cells from obese mice so that we may target T-cell metabolism to improve immune response to infection.
Methods
Mice were fed normal chow or high-fat diet for 18–19 weeks. Changes in T-cell populations were analyzed in both adipose tissue and spleens using flow cytometry. Splenic T cells were further analyzed for nutrient uptake and extracellular metabolic flux. As changes in T-cell mitochondrial oxidation were observed in obesity, obese mice were treated with metformin for 6 weeks and compared to lean control mice or obese mice undergoing weight loss through diet switch; immunity was measured by survival to influenza infection.
Results
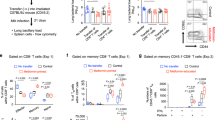
We found changes in T-cell populations in adipose tissue of high-fat diet-induced obese mice, characterized by decreased proportions of Treg cells and increased proportions of CD8+ T cells. Activated CD4+ T cells from obese mice had increased glucose uptake and oxygen consumption rate (OCR), compared to T cells from lean controls, indicating increased mitochondrial oxidation of glucose. Treatment of isolated CD4+ T cells with metformin was found to inhibit OCR in vitro and alter the expression of several activation markers. Last, treatment of obese mice with metformin, but not weight loss, was able to improve survival to influenza in obesity.
Conclusions
T cells from obese mice have an altered metabolic profile characterized by increased glucose oxidation, which can be targeted to improve survival against influenza infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17.
Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21–33.
Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13:981–1000.
Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7:66–75.
Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–16.
Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS ONE. 2010;5:e9694.
Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis. 2011;52:301–12.
Neidich SD, Green WD, Rebeles J, Karlsson EA, Schultz-Cherry S, Noah TL, et al. Increased risk of influenza among vaccinated adults who are obese. Int J Obes. 2017;41:1324–30.
Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes. 2012;36:1072–7.
Rebeles J, Green WD, Alwarawrah Y, Nichols AG, Eisner W, Danzaki K, et al. Obesity-induced changes in T-cell metabolism are associated with impaired memory T-cell response to influenza and are not reversed with weight loss. J Infect Dis. 2019;219:1652–61.
Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–43.
Smith AG, Sheridan PA, Tseng RJ, Sheridan JF, Beck MA. Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus. Immunology. 2009;126:268–79.
Green WD, Beck MA. Obesity impairs the adaptive immune response to influenza virus. Ann Am Thorac Soc. 2017;14 Suppl 5:S406–9.
Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–34.
Alwarawrah Y, Kiernan K, MacIver NJ. Changes in nutritional status impact immune cell metabolism and function. Front Immunol. 2018;9:1055.
MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–83.
Lochner M, Berod L, Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 2015;36:81–91.
Ma EH, Bantug G, Griss T, Condotta S, Johnson RM, Samborska B, et al. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 2017;25:345–57.
Ren W, Liao Y, Ding X, Jiang Y, Yan J, Xia Y, et al. Slc6a13 deficiency promotes Th17 responses during intestinal bacterial infection. Mucosal Immunol. 2019;12:531–44.
Lee CF, Lo YC, Cheng CH, Furtmuller GJ, Oh B, Andrade-Oliveira V, et al. Preventing allograft rejection by targeting immune metabolism. Cell Rep. 2015;13:760–70.
Milner JJ, Rebeles J, Dhungana S, Stewart DA, Sumner SC, Meyers MH, et al. Obesity increases mortality and modulates the lung metabolome during pandemic H1N1 influenza virus infection in mice. J Immunol. 2015;194:4846–59.
Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–7.
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–85.
Paich HA, Sheridan PA, Handy J, Karlsson EA, Schultz-Cherry S, Hudgens MG, et al. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity. 2013;21:2377–86.
Chapman NM, Boothby MR, Chi H. Metabolic coordination of T cell quiescence and activation. Nat Rev Immunol. 2020;20:55–70.
Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol. 2014;192:136–44.
Gerriets VA, Danzaki K, Kishton RJ, Eisner W, Nichols AG, Saucillo DC, et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur J Immunol. 2016;46:1970–83.
Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PloS ONE. 2012;7:e46057.
Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20:61–72.
Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–46.
Wieman HL, Horn SR, Jacobs SR, Altman BJ, Kornbluth S, Rathmell JC. An essential role for the Glut1 PDZ-binding motif in growth factor regulation of Glut1 degradation and trafficking. Biochem J. 2009;418:345–67.
Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6.
Hengel RL, Thaker V, Pavlick MV, Metcalf JA, Dennis G Jr., Yang J, et al. Cutting edge: L-selectin (CD62L) expression distinguishes small resting memory CD4+ T cells that preferentially respond to recall antigen. J Immunol. 2003;170:28–32.
Baaten BJ, Li CR, Bradley LM. Multifaceted regulation of T cells by CD44. Commun Integr Biol. 2010;3:508–12.
Hufford MM, Kim TS, Sun J, Braciale TJ. The effector T cell response to influenza infection. Curr Top Microbiol Immunol. 2015;386:423–55.
Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol. 2010;184:3127–33.
Funding
This work was funded by NIH R01-DK106090, the Translating Duke Health: Controlling the Immune System Initiative, and the Derfner Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Alwarawrah, Y., Nichols, A.G., Green, W.D. et al. Targeting T-cell oxidative metabolism to improve influenza survival in a mouse model of obesity. Int J Obes 44, 2419–2429 (2020). https://doi.org/10.1038/s41366-020-00692-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-00692-3
This article is cited by
-
Emerging mechanisms of obesity-associated immune dysfunction
Nature Reviews Endocrinology (2024)
-
Irgm1 regulates metabolism and function in T cell subsets
Scientific Reports (2022)
-
Microenvironmental influences on T cell immunity in cancer and inflammation
Cellular & Molecular Immunology (2022)
-
Bioequivalence and Safety Assessment of Two Formulations of Metformin Hydrochloride Sustained-Release Tablets (Yuantang® SR and Glucophage® XR) Under Fed Conditions in Healthy Chinese Adult Subjects: An Open-Label, Two-Way Crossover, Sequence Randomized Phase I Clinical Trial
Drugs in R&D (2022)
-
Bacterial factors required for Streptococcus pneumoniae coinfection with influenza A virus
Journal of Biomedical Science (2021)