Abstract
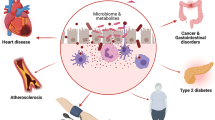
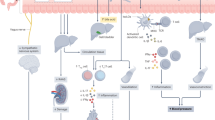
The co-existence of humans and gut microbiota started millions of years ago. Until now, a balance gradually developed between gut bacteria and their hosts. It is now recognized that gut microbiota are key to form adequate immune and metabolic functions and, more in general, for the maintenance of good health. Gut microbiota are established before birth under the influence of maternal nutrition and metabolic status, which can impact the future metabolic risk of the offspring in terms of obesity, diabetes, and cardiometabolic disorders during the lifespan. Obesity and diabetes are prone to disrupt the gut microbiota and alter the gut barrier permeability, leading to metabolic endotoxaemia with its detrimental consequences on health. Specific bacterial sequences are now viewed as peculiar signatures of the metabolic syndrome across life stages in each individual, and are linked to pathogenesis of cardiovascular diseases (CVDs) via metabolic products (metabolites) and immune modulation. These mechanisms have been linked, in association with abnormalities in microbial richness and diversity, to an increased risk of developing arterial hypertension, systemic inflammation, nonalcoholic fatty liver disease, coronary artery disease, chronic kidney disease, and heart failure. Emerging strategies for the manipulation of intestinal microbiota represent a promising therapeutic option for the prevention and treatment of CVD especially in individuals prone to CV events.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
World Health Organization (WHO). Fact-sheets on cardiovascular disease. World Health Organization; Geneva, Switzerland, 2017. https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
Bäckhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12:611–22.
Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15:630–8.
Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80.
Natividad JMM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res. 2013;69:42–51.
Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–44.
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40.
Zhang M, Chekan JR, Dodd D, Hong P-Y, Radlinski L, Revindran V, et al. Xylan utilization in human gut commensal bacteria is orchestrated by unique modular organization of polysaccharide-degrading enzymes. Proc Natl Acad Sci. 2014;111:E3708–17.
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7.
Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72.
Bhat MI, Kapila R. Dietary metabolites derived from gut microbiota: critical modulators of epigenetic changes in mammals. Nutr Rev. 2017;75:374–89.
Everard A, Geurts L, Van Roye M, Delzenne NM, Cani PD. Tetrahydro iso-alpha acids from hops improve glucose homeostasis and reduce body weight gain and metabolic endotoxemia in high-fat diet-fed mice. PLoS ONE. 2012;7:e33858.
Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83.
Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–7.
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;26:55–60.
De Lorenzo A. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol. 2016;22:681.
Abenavoli L, Luigiano C, Guzzi PH, Milic N, Morace C, Stelitano L, et al. Serum adipokine levels in overweight patients and their relationship with non-alcoholic fatty liver disease. Panminerva Med. 2014;56:189–93.
Després J-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–13.
Alwardat N, Di Renzo L, de Miranda RC, Alwardat S, Sinibaldi Salimei P, De Lorenzo A. Association between hypertension and metabolic disorders among elderly patients in North Jordan. Diabetes Metab Syndr. 2018;12:661–6.
Shah A, Mehta N, Reilly MP. Adipose inflammation, insulin resistance, and cardiovascular disease. J Parenter Enter Nutr. 2008;32:638–44.
Karelis AD, Faraj M, Bastard J-P, St-Pierre DH, Brochu M, Prud’homme D, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–50.
Chang Y, Kim B-K, Yun KE, Cho J, Zhang Y, Rampal S, et al. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014;63:2679–86.
Camhi SM, Crouter SE, Hayman LL, Must A, Lichtenstein AH. Lifestyle behaviors in metabolically healthy and unhealthy overweight and obese women: a preliminary study. PLoS ONE. 2015;10:e0138548.
Oliverosa E, Somersa VK, Sochora O, Goela K, Lopez-Jimeneza F. The concept of normal weight obesity. Prog Cardiovas Dis. 2014;56:426–33.
Marques-Vidal P, Pecoud A, Hayoz D, et al. Prevalence of normal weight obesity in Switzerland: effect of various definitions. Eur J Nutr. 2008;47:251–7.
Lee S-H, Han K, Yang HK, Kim H-S, Cho J-H, Kwon H-S, et al. A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr Diabetes. 2015;5:e149.
Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713.
De Lorenzo A, Martinoli R, Vaia F, Di Renzo L. Normal weight obese (NWO) women: an evaluation of a candidate new syndrome. Nutr Metab Cardiovas Dis. 2006;16:513–23.
Di Renzo L, Del Gobbo V, Bigioni M, Premrov MG, Cianci R, De Lorenzo A. Body composition analyses in normal weight obese women. Eur Rev Med Pharmacol Sci. 2006;10:191–6.
De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L. Normal-weight obese syndrome: early inflammation? Am J Clin Nutr. 2007;85:40–5.
Di Renzo L, Galvano F, Orlandi C, Bianchi A, Di Giacomo C, La Fauci L, et al. Oxidative stress in normal-weight obese syndrome. Obesity. 2010;18:2125–30.
Di Renzo L, Sarlo F, Petramala L, Iacopino L, Monteleone G, Colica C, et al. Association between -308 G/A TNF- α polymorphism and appendicular skeletal muscle mass index as a marker of sarcopenia in normal weight obese syndrome. Dis Markers. 2013;35:615–23. https://doi.org/10.1155/2013/983424.
Di Renzo L, Gratteri S, Sarlo F, Cabibbo A, Colica C, De Lorenzo A. Individually tailored screening of susceptibility to sarcopenia using p53 codon 72 polymorphism, phenotypes, and conventional risk factors. Dis Markers. 2014;2014:1–10.
Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–46.
Marques-Vidal P, Pécoud A, Hayoz D, Paccaud F, Mooser V, Waeber G, et al. Normal weight obesity: relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutr Metab Cardiovasc Dis. 2010;20:669–75.
Di Renzo L, Tyndall E, Gualtieri P, Carboni C, Valente R, Ciani AS, et al. Association of body composition and eating behavior in the normal weight obese syndrome. Eat Weight Disord 2016;21:99–106.
De Lorenzo A, Costacurta M, Merra G, Gualtieri P, Cioccoloni G, Marchetti M, et al. Can psychobiotics intake modulate psychological profile and body composition of women affected by normal weight obese syndrome and obesity? A double blind randomized clinical trial. J Transl Med. 2017;15:135.
Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta. 2009;1790:1117–23.
Bays H. Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014;21:345–51.
Jokela M, Hintsanen M, Hakulinen C, Batty GD, Nabi H, Singh-Manoux A, et al. Association of personality with the development and persistence of obesity: a meta-analysis based on individual-participant data. Obes Rev. 2013;14:315–23.
Däbritz J, Menheniott TR. Linking immunity, epigenetics, and cancer in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1638–54.
Harris RA, Nagy-Szakal D, Pedersen N, Opekun A, Bronsky J, Munkholm P, et al. Genome-wide peripheral blood leukocyte DNA methylation microarrays identified a single association with inflammatory bowel diseases. Inflamm Bowel Dis. 2012;18:2334–41.
Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem. 2012;23:853–9.
Remely M, Lovrecic L, de la Garza AL, Migliore L, Peterlin B, Milagro FI, et al. Therapeutic perspectives of epigenetically active nutrients: therapeutic epigenetically active nutrients. Br J Pharmacol. 2015;172:2756–68.
Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011;6:93–108.
Scarpa M, Stylianou E. Epigenetics: concepts and relevance to IBD pathogenesis. Inflamm Bowel Dis. 2012;18:1982–96.
Krautkramer KA, Kreznar JH, Romano KA, Vivas EI, Barrett-Wilt GA, Rabaglia ME, et al. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol Cell. 2016;64:982–92.
Eeckhaut V, Ducatelle R, Sas B, Vermeire S, Van Immerseel F. Progress towards butyrate-producing pharmabiotics: Butyricicoccus pullicaecorum capsule and efficacy in TNBS models in comparison with therapeutics. Gut. 2014;63:367.
Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease. J Am Coll Cardiol. 2009;53:1925–32.
Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes. 2016;2016:1–27.
Tilg H, Moschen AR, Kaser A. Obesity and the microbiota. Gastroenterology. 2009;136:1476–83.
Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes. 2008;32:S7–12.
Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–23.
Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012;7:91–109. https://doi.org/10.2217/fmb.11.142
Beynon AL, Brown MR, Wright R, Rees MI, Sheldon IM, Davies JS. Ghrelin inhibits LPS-induced release of IL-6 from mouse dopaminergic neurones. J Neuroinflammation. 2013;10:40.
Avolio E, Gualtieri P, Romano L, Pecorella C, Ferraro S, Di Renzo L, et al. Obesity and body composition in man and woman: associated diseases and new role of gut microbiota. Curr Med Chem. 2020;27:216-29.
Graessler J, Bornstein TD, Goel D, Bhalla VP, Lohmann T, Wolf T, et al. Lipidomic profiling before and after Roux-en-Y gastric bypass in obese patients with diabetes. Pharmacogenomics J. 2014;14:201–7.
Nyangale EP, Mottram DS, Gibson GR. Gut microbial activity, implications for health and disease: the potential role of metabolite analysis. J Proteome Res. 2012;11:5573–85.
Respondek F, Gerard P, Bossis M, Boschat L, Bruneau A, Rabot S, et al. Short-chain fructo-oligosaccharides modulate intestinal microbiota and metabolic parameters of humanized gnotobiotic diet induced obesity mice. PLoS ONE. 2013;8:e71026.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4.
Furet J-P, Kong L-C, Tap J, Poitou C, Basdevant A, Bouillot J-L, et al. Differential adaptation of human gut microbiota to bariatric surgery–induced weight loss. Diabetes. 2010;59:3049–57.
Kong L-C, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot J-L, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24.
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–70.
Aron-Wisnewsky J, Prifti E, Belda E, Ichou F, Kayser BD, Dao MC, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2019;68:70–82.
Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–8.
Wang F-G, Bai R-X, Yan W-M, Yan M, Dong L-Y, Song M-M. Differential composition of gut microbiota among healthy volunteers, morbidly obese patients and post-bariatric surgery patients. Exp Ther Med. 2019;17:2268–78.
Larsen N, Vogensen FK, van den Berg FWJ, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5:e9085.
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–17.
Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103.
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72.
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–71.
Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Muccioli GM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–86.
Karlsson CLJ, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity. 2012;20:2257–61.
Shin N-R, Lee J-C, Lee H-Y, Kim M-S, Whon TW, Lee M-S, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–35.
Ghazalpour A, Cespedes I, Bennett BJ, Allayee H. Expanding role of gut microbiota in lipid metabolism. Curr Opin Lipidol. 2016;27:141–7.
Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51:1101–12.
Fu J, Bonder MJ, Cenit MC, Tigchelaar EF, Maatman A, Dekens JAM, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117:817–24.
Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall H-U, Bamberg K, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–35.
Zhang Y, Ge X, Heemstra LA, Chen W-D, Xu J, Smith JL, et al. Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol Endocrinol. 2012;26:272–80.
Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77.
Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes. 2016;2016:7353642.
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63.
Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37.
Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, et al. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015;10:326–38.
Kang Y, Cai Y. Gut microbiota and hypertension: from pathogenesis to new therapeutic strategies. Clin Res Hepatol Gastroenterol. 2018;42:110–7.
Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens. 2015;24:403–9.
Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110:4410–5.
Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5:202–7.
Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016;5:e003698.
Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA. 2011;108:8030–5.
Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, et al. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2017;120:312–23.
Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31:817–44.
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–9.
Tang WHW, Hazen SL. Microbiome, trimethylamine N-oxide, and cardiometabolic disease. Transl Res. 2017;179:108–15.
Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–40.
Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genom. 2017;49:96–104.
Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, et al. Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension. 2016;67:469–74.
Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, et al. High fibre diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in DOCA-salt hypertensive mice. Circulation. 2017;135:964–77.
Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C, et al. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol. 2017;7:381
Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14.
Duseja A, Chawla YK. Obesity and NAFLD: the role of bacteria and microbiota. Clin Liver Dis. 2014;18:59–71.
Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–87.
Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–94.
Yao C, Muir J, Gibson P. Insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther. 2016;43:181–96.
Chen YM, Liu Y, Zhou RF, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with nonalcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076.
Mollica MP, Mattace Raso G, Cavaliere G, Trinchese G, De Filippo C, Aceto S, et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes. 2017 ;66:1405–18.
den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–408.
Kondo T, Kishi M, Fushimi T, Kaga T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J Agric Food Chem. 2009;57:5982–6.
Sakakibara S, Yamauchi T, Oshima Y, Tsukamoto Y, Kadowaki T. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A (y) mice. Biochem Biophys Res Commun. 2006;344:597–604.
Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118:535–46.
Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20th century: coronary heart disease. Am J Med. 2014;127:807–12.
Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108:4592–8.
Calandrini C, Ribeiro A, Gonnelli A, Ota-Tsuzuki C, Rangel L, Saba-Chujfi E, et al. Microbial composition of atherosclerotic plaques. Oral Dis. 2014;20:e128–34.
Ma G, Pan B, Chen Y, Guo C, Zhao M, Zheng L, et al. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci Rep. 2017;37:BSR20160244.
Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–24.
Wu ZX, Su-Fang L, Hong C, Jun-Xian S, Yuan-Feng G, Feng Z, et al. The changes of gut microbiota after acute myocardial infarction in rats. PLoS ONE. 2017;12:e0180717.
Yamashita T, Emoto T, Sasaki N, Hirata K. Gut microbiota and coronary artery disease. Int Heart J. 2016;57:9.
De Nicola L, Minutolo R. Worldwide growing epidemic of CKD: fact or fiction? Kidney Int. 2016;90:482–4.
Bruck K, Stel VS, Gambaro G, Hallan S, Volzke H, Arnlov J, et al. CKD prevalence varies across the european general population. J Am Soc Nephrol. 2016;27:2135–47.
Castillo-Rodriguez E, Fernandez-Prado R, Esteras R, Perez-Gomez MV, Gracia-Iguacel C, Fernandez-Fernandez B, et al. Impact of altered intestinal microbiota on chronic kidney disease progression. Toxins. 2018;10:E300.
Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M. European Uremic Toxin Work, G, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–8.
Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69:1081–7.
Missailidis C, Hallqvist J, Qureshi AR, Barany P, Heimburger O, Lindholm B, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS ONE. 2016;11:e0141738.
Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010–6.
Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. 2016;67:483–98.
Nomura S, et al. Genetic and non-genetic determinants of clinical phenotypes in cardiomyopathy. J Cardiol. 2019;73:187–90.
Zhernakova DV, Le TH, Kurilshikov A, Atanasovska B, Bonder MJ, Sanna S, et al. LifeLines cohort study; BIOS consortium. Individual variations in cardiovascular-disease-related protein levels are driven by genetics and gut microbiome. Nat Genet. 2018;50:1524–32.
Aguiar CJ, Rocha-Franco JA, Sousa PA, Santos AK, Ladeira M, Rocha-Resende C, et al. Succinate causes pathological cardiomyocyte hypertrophy through GPR91 activation. Cell Commun Signal. 2014;12:78.
Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J. 2010;31:1771–9.
Troseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717–26.
Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–9.
Kamo T, Akazawa H, Suzuki JI, Komuro I. Novel concept of a heart-gut axis in the pathophysiology of heart failure. Korean Circ J. 2017;47:663–9.
Peschel T, Schonauer M, Thiele H, Anker SD, Schuler G, Niebauer J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail. 2003;5:609–14.
Sandek A, Bjarnason I, Volk HD, Crane R, Meddings JB, Niebauer J, et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol. 2012;157:80–85.
Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4:220–7.
Luedde M, Winkler T, Heinsen FA, Rühlemann MC, Spehlmann ME, Bajrovic A, et al. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail. 2017;4:282–90.
Kummen M, Mayerhofer CCK, Vestad B, Broch K, Awoyemi A, Storm-Larsen C, et al. Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J Am Coll Cardiol. 2018;71:1184–6.
Kamo T, Akazawa H, Suda W, Saga-Kamo A, Shimizu Y, Yagi H, et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS ONE. 2017;12:e0174099.
Cui X, Ye L, Li J, Jin L, Wang W, Li S, et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. 2018;8:635.
Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, et al. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide. exacerbate pressure overload-induced heart failure. Circ Heart Fail. 2016;9:e002314.
Sun X, Jiao X, Ma Y, Liu Y, Zhang L, He Y, et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun. 2016;481:63–70.
Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, et al. Non-lethal Inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–95.
Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Tang WH, et al. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc. 2016;5:e002816.
Senthong V, Wang Z, Fan Y, Wu Y, Hazen SL, Tang WH. Trimethylamine N-oxide and mortality risk in patients with peripheral artery disease. J Am Heart Assoc. 2016;5:e004237.
Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–14.
Reiner MF, Müller D, Gobbato S, Stalder O, Limacher A, Bonetti NR, et al. Gut microbiota-dependent trimethylamine-N-oxide (TMAO) shows a U-shaped association with mortality but not with recurrent venous thromboembolism. Thromb Res. 2019;174:40–7.
Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc. 2017;6:e004947.
Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. 2017;38:2948–56.
Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, et al. Trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS ONE. 2014;9:e114969.
Perez PF, Donnet-Hughes A, Schrffrin Schiffrin EJ, Marteau P, Brassart D. Presentation of microbial signals via maternal cells. an evolutionary advantage of mammals. In: Intestinal microbiota in health and disease. Modern concepts. Boca Raton, FL (USA). CRC Press; 2014. EJ
Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129.
Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra265 37.
Tun HM, Bridgman SL, Chari R, Field CJ, Guttman DS, Becker AB. Canadian Healthy Infant Longitudinal Development (CHILD) Study Investigators, et al. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr. 2018;172:368–77.
Stanislawski MA, Dabelea D, Wagner BD, Iszatt N, Dahl C, Sontag MK, et al. Gut microbiota in the first 2 years of life and the association with body mass index at age 12 in a norwegian birth cohort. MBio. 2018;9:e01751-18.
Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65.
Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111:2247–52.
Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory t cells by suppression of histone deacetylases and regulation of the mtor-s6k pathway. Mucosal Immunol. 2015;8:80–93.
Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Thakali KM, et al. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS ONE. 2017;12:e0175675.
Bruce-Keller AJ, Fernandez-Kim SO, Townsend RL, Kruger C, Carmouche R, Newman S, et al. Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS ONE. 2017;12:e0175577.
Luoto R, Laitinen K, Nermes M, Isolauri E Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr. 2010;103:1792–9.
Borewicz K, Suarez-Diez M, Hechler C, Beijers R, de Weerth C, Arts I, et al. The effect of prebiotic fortified infant formulas on microbiota composition and dynamics in early life. Sci Rep. 2019;9:2434.
van Oostrom AJ, Rabelink TJ, Verseyden C, et al. Activation of leuko- cytes by postprandial lipemia in healthy volunteers. Atherosclerosis. 2004;177:175–82.
Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–92.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63.
De Lorenzo A, Bernardini S, Gualtieri P, Cabibbo A, Perrone MA, Giambini I, et al. Mediterranean meal versus Western meal effects on postprandial ox-LDL, oxidative and inflammatory gene expression in healthy subjects: a randomized controlled trial for nutrigenomic approach in cardiometabolic risk. Acta Diabetol. 2016;54:141–9.
Calabrese V, Cornelius C, Trovato A, Cavallaro M, Mancuso C, Di Renzo L, et al. The hormetic role of dietary antioxidants in free radical-related diseases. Curr Pharm Des. 2010;16:877–83.
Di Daniele N, Petramala L, Di Renzo L, Sarlo F, Della Rocca DG, Rizzo M, et al. Body composition changes and cardiometabolic benefits of a balanced Italian Mediterranean Diet in obese patients with metabolic syndrome. Acta Diabetol. 2012;50:409–16.
De Lorenzo A, Noce A, Bigioni M, Calabrese V, Della Rocca DG, Di Daniele N, et al. The effects of Italian Mediterranean organic diet (IMOD) on health status. Curr Pharm Des. 2010;16:814–24.
Maffeis C, Pinelli L, Surano MG, Fornari E, Cordioli S, Gasperotti S. Pro-atherogenic postprandial profile: meal-induced changes of lipoprotein sub-fractions and in-flammation markers in obese boys. Nutr Metab Cardiovasc Dis. 2012;22:959–65.
Di Renzo L, Marsella LT, Carraro A, Valente R, Gualtieri P, Gratteri S, et al. Changes in LDL oxidative status and oxidative and inflammatory gene expression after red wine intake in healthy people: a randomized trial. Mediators Inflamm. 2015;2015:317348.
Di Renzo L, Merra G, Botta R, Gualtieri P, Manzo A, Perrone MA, et al. Post-prandial effects of hazelnut-enriched high fat meal on LDL oxidative status, oxidative and inflammatory gene expression of healthy subjects: a randomized trial. Eur Rev Med Pharmacol Sci. 2017;21:1610–26.
Di Renzo L, Carraro A, Valente R, Iacopino L, Colica C, De Lorenzo A. Intake of red wine in different meals modulates oxidized LDL level, oxidative and inflammatory gene expression in healthy people: a randomized crossover trial. Oxid Med Cell Longev. 2014;2014:681318.
Di Renzo L, Cioccoloni G, Sinibaldi Salimei P, Ceravolo I, De Lorenzo A, Gratteri S. Alcoholic beverage and meal choices for the prevention of noncommunicable diseases: a randomized nutrigenomic trial. Oxid Med Cell Longev. 2018:5461436.
Di Renzo L, Di Pierro D, Bigioni M, Sodi V, Galvano F, Cianci R, et al. Is antioxidant plasma status in humans a consequence of the antioxidant food content influence? Eur Rev Med Pharmacol Sci. 2007;11:185–92.
Soldati L, Di Renzo L, Jirillo E, Ascierto PA, Marincola FM, De Lorenzo A. The influence of diet on anti-cancer immune responsiveness. J Transl Med. 2018;16:75.
Mocchegiani E, Costarelli L, Giacconi R, Piacenza F, Basso A. Malavolta R. Zinc, metallothioneins and immunosenescence: effect of zinc supply as nutrigenomic approach. Biogerontology. 2011;12:455–65.
Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85.
Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. 2014;64:897–903.
Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–50.
Daubioul C, Horsmans Y, Lambert P, Danse E, Delzenne NM. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: results of a pilot study. Eur J Clin Nutr. 2005;59:723–6.
Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545–53.
Ong HS, Yim HCH. Microbial factors in inflammatory diseases and cancers. Adv Exp Med Biol. 2017;1024:153–74.
Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1–12.
Kelly D, King T, Aminov R. Importance of microbial colonization of the gut in early life to the development of immunity. Mutat Res. 2007;622:58–69.
Colica C, Di Renzo L, Trombetta D, Smeriglio A, Bernardini S, Cioccoloni G, et al. Antioxidant effects of a hydroxytyrosol-based pharmaceutical formulation on body composition, metabolic state, and gene expression: a randomized double-blinded, placebo-controlled crossover trial. Oxid Med Cell Longev. 2017;2017:2473495.
Bachmeier BE, Mohrenz IV, Mirisola V, Schleicher E, Romeo F, Hohneke C, et al. Curcumin downregulates the inflammatory cytokines CXCL1 and-2 in breast cancer cells via NFkB. Carcinogenesis. 2008;29:779–89.
Kawaguchi K, Matsumoto T, Kumazawa Y. Effects of antioxidant polyphenols on TNF-alpha-related diseases. Curr Topo Med Chem. 2011;11:1767–679.
Tsao R, Li H. Antioxidant properties in vitro and in vivo: realistic assessments of efficacy of plant extracts. CAB Rev. 2012;7:9.
Scott KP, Antoine J-M, Midtvedt T, van Hemert S. Manipulating the gut microbiota to maintain health and treat disease. Microb Ecol Health Dis. 2015;26:25877.
Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–80.
McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1–48.
Liubakka A, Vaughn BP. Clostridium difficile Infection and fecal microbiota transplant. AACN Adv Crit Care. 2016;27:324–37.
Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–18.
Mangiola F. Gut microbiota in autism and mood disorders. World J Gastroenterol. 2016;22:361.
Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–60.
de Groot PF, Frissen MN, de Clercq NC, Nieuwdorp M. Fecal microbiota transplantation in metabolic syndrome: history, present and future. Gut Microbes. 2017;8:253–67.
Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation. 2016;133:2434–46.
Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman J, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6.e7.
Acknowledgements
Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group members served as collaborators and approved the final version of the manuscript: Colao Annamaria, Savastano Silvia, Barrea Luigi, Muscogiuri Giovanna, Alviggi Carlo, Angrisani Luigi, Annunziata Giuseppe, Beguinot Francesco, Belfiore Annamaria, Belfiore Antonino, Bellastella Giuseppe, Biondi Bernadette, Bonaduce Domenico, Bordoni Laura, Brasacchio Caterina, Capaldo Brunella, Caprio Massimiliano, Cataldi Mauro, Cignarelli Angelo, Cittadini Antonello, Conforti Alessandro, Cuomo Rosario, De Placido Giuseppe, De Siena Marina, Di Carlo Costantino, Di Luigi Luigi, Di Nisio Andrea, Di Renzo Laura, Di Somma Carolina, Docimo Ludovico, Donini Lorenzo Maria, Federici Massimo, Foresta Carlo, Gabbianelli Rosita, Gambineri Alessandra, Gastaldelli Amalia, Giallauria Francesco, Giardiello Cristiano, Gnessi Lucio, Guida Brunella, Laudisio Daniela, Lenzi Andrea, Macchia Paolo Emidio, Manno Emilio, Marzullo Paolo, Migliaccio Silvia, Muratori Fabrizio, Musella Mario, Nardone Gerardo, Nicasto Vincenzo, Piazza Luigi, Pilone Vincenzo, Pivari Francesca, Pivonello Rosario, Pugliese Gabriella, Riccardi Gabriele, Ritieni Alberto, Salzano Ciro, Sanduzzi Alessandro, Sbraccia Paolo, Sesti Giorgio, Soldati Laura, Taglialatela Maurizio, Trimarco Bruno, Tuccinardi Dario.
Funding
The 2019 OPERA meeting was organized by Panta Rei Srl and sponsored by Novo Nordisk, Therascience, Bruno Pharma, Merck, Savio Pharma Italia Srl, IBSA Institut Biochimique SA, Bioitalia Srl, Cohesion Pharmaceutical, and Specchiasol Srl. Publication of this article as part of a supplement was sponsored by Panta Rei Srl, Naples, Italy. The meeting sponsors and organizer did not have access to the manuscripts and the authors maintained control of the content.
Author information
Authors and Affiliations
Consortia
Contributions
PM and LDR equally contributed to the work. The authors’ responsibilities were as follows: PM, LDR, GP, MDS, LB, GM, and SS were responsible for the concept of this paper and drafted the manuscript; GM, AC, and SS provided a critical review of the paper. OPERA Group members participated to the revision of the manuscript. All authors and OPERA Group Members contributed to and agreed on the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marzullo, P., Di Renzo, L., Pugliese, G. et al. From obesity through gut microbiota to cardiovascular diseases: a dangerous journey. Int J Obes Supp 10, 35–49 (2020). https://doi.org/10.1038/s41367-020-0017-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41367-020-0017-1
This article is cited by
-
Mediterranean Diet and Obesity-related Disorders: What is the Evidence?
Current Obesity Reports (2022)
-
The impact of gut microbiota metabolites on cellular bioenergetics and cardiometabolic health
Nutrition & Metabolism (2021)
-
Spot-light on microbiota in obesity and cancer
International Journal of Obesity (2021)