Abstract
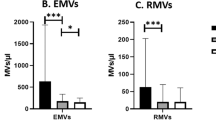
Rather than being mere biomarkers reflecting generalized vascular injury, endothelial- (EMVs) and platelet-derived (PMVs) microvesicles have emerged as potent regulators of intercellular communication with significant biologic effects in vascular homeostasis and several pathophysiological responses including inflammation and thrombosis. So far, studies in hypertension are scarce, whereas no studies exist in masked hypertension (MH). We measured EMVs and PMVs in untreated, newly diagnosed hypertensives (HTs) and MHs compared to normotensive controls (NTs), and associated them with various cardiovascular risk factors. Sustained hypertension (SHT) and MH were defined according to standard blood pressure (BP) criteria. All HTs were free of cardiovascular disease and medications. Microvesicles’ quantitation and detection were performed by flow cytometry by using cell-specific antibodies and corresponding isotypes (anti-CD105 and anti-CD144 for EMVs, anti-CD42a for PMVs, and Annexin V–fluorescein isothiocyanate for all microvesicles). In this study, we included 59 HTs (44 SHTs and 15 MHs) and 27 NTs. HTs had significantly elevated EMVs (p = 0.004), but not PMVs compared to NTs. MHs had significantly elevated EMVs compared to NTs (p = 0.012) but not compared to SHTs. Furthermore, EMVs significantly correlated with ambulatory (r = 0.214–0.284), central BP (r = 0.247–0.262), and total vascular resistance (r = 0.327–0.361). EMVs are increased not only in SHTs but also in MHs, a hypertension phenotype with a cardiovascular risk close to SHT. EMVs have emerged as active contributors to thromboinflammation and vascular damage and may explain, in part, the adverse cardiovascular profile of SHTs and MHs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Arraud N, Linares R, Tan S, Gounou C, Pasquet JM, Mornet S, et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12:614–27.
Ridger V, Boulanger C, Angelillo-Scherrer A, Badimon L, Blanc-Brude O, Bochaton-Piallat M, et al. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb Haemost. 2017;117:1296–316.
Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–57.
Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clin Sci. 2013;124:423–41.
Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33.
Leroyer AS, Anfosso F, Lacroix R, Sabatier F, Simoncini S, Njock SM, et al. Endothelial-derived microparticles: biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb Haemost. 2010;104:456–63.
Sinning JM, Losch J, Walenta K, Böhm M, Nickenig G, Werner N. Circulating CD31 +/Annexin V + microparticles correlate with cardiovascular outcomes. Eur Heart J. 2011;32:2034–41.
Yong PJA, Koh CH, Shim WSN. Endothelial microparticles: missing link in endothelial dysfunction? Eur J Prev Cardiol. 2013;20:496–512.
Aatonen M, Grönholm M, Siljander PM. Platelet-derived microvesicles: multitalented participants in intercellular communication. Semin Thromb Hemost. 2012;38:102–13.
Melki I, Tessandier N, Zufferey A, Boilard E. Platelet microvesicles in health and disease. Platelets. 2017;28:214–21.
Amabile N, Rautou PE, Tedgui A, Boulanger CM. Microparticles: key protagonists in cardiovascular disorders. Semin Thromb Hemost. 2010;1:907–16.
Voukalis C, Shantsila E, Lip GYH. Microparticles and cardiovascular diseases. Ann Med. 2019;51:193–223.
Gkaliagkousi E, Gavriilaki E, Yiannaki E, Vasileiadis I, Nikolaidou B, Lazaridis A, et al. Platelet microvesicles are associated with the severity of coronary artery disease: comparison between peripheral and coronary circulation. J Thromb Thrombolysis. 2020.
Gkaliagkousi E, Nikolaidou B, Gavriilaki E, Lazaridis A, Yiannaki E, Anyfanti P, et al. Increased erythrocyte- and platelet-derived microvesicles in newly diagnosed type 2 diabetes mellitus. Diabetes Vasc Dis Res. 2019;16:458–65.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. ESC/ESH Guidelines for the management of arterial hypertension. J Hypertens. 2018;36:1953–2041.
Gkaliagkousi E, Corrigall V, Becker S, De WP, Shah A, Zamboulis C, et al. Decreased platelet nitric oxide contributes to increased circulating monocyte-platelet aggregates in hypertension. Eur Hear J. 2009;30:3048–54.
Gkaliagkousi E, Gavriilaki E, Triantafyllou A, Douma S. Clinical significance of endothelial dysfunction in essential hypertension. Curr Hypertens Rep. 2015;17:85.
Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, et al. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–7.
Stockelman KA, Hijmans JG, Bammert T, Greiner J, Stauffer BL, DeSouza CA. Circulating endothelial cell derived microvesicles are elevated with hypertension and associated with endothelial dysfunction. Can J Physiol Pharmacol. 2020;98:557–61.
Sansone R, Baaken M, Horn P, Schuler D, Westenfeld R, Amabile N, et al. Release of endothelial microparticles in patients with arterial hypertension, hypertensive emergencies and catheter-related injury. Atherosclerosis. 2018;273:67–74.
Hu S, Zhang H, Zhang Q, Xiu R. CD144 + EMPs / CD62E + EMPs: a couple of new biomarkers to monitor endothelial function in hypertension with hyperlipidemia involved. Int J Cardiol. 2014;175:203.
Robert S, Poncelet P, Lacroix R. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost. 2009;7:190–7.
Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360.
Berezin A, Zulli A, Kerrigan S, Petrovic D, Kruzliak P. Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin Biochem. 2015;48:562–8.
Tomaniak M, Gąsecka A, Filipiak KJ. Cell-derived microvesicles in cardiovascular diseases and antiplatelet therapy monitoring—a lesson for future trials? Current evidence, recent progresses and perspectives of clinical application. Int J Cardiol. 2017;226:93–102.
Bernal-Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, et al. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145:962–70.
El-Gamal H, Parray AS, Mir FA, Shuaib A, Agouni A. Circulating microparticles as biomarkers of stroke: a focus on the value of endothelial- and platelet-derived microparticles. J Cell Physiol. 2019;234:16739–54.
Li S, Wei J, Zhang C, Li X, Meng W, Mo X, et al. Cell-derived microparticles in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Cell Physiol Biochem. 2016;39:2439–50.
Suades R, Padró T, Alonso R, Mata P, Badimon L. Lipid-lowering therapy with statins reduces microparticle shedding from endothelium, platelets and inflammatory cells. Thromb Haemost. 2013;110:366–77.
Bulut D, Becker V, Mügge A. Acetylsalicylate reduces endothelial and platelet-derived microparticles in patients with coronary artery disease. Can J Physiol Pharmacol. 2011;89:239–44.
Danielle Tientcheu C, Ayers SR, Das DK, McGuire JA, de L, Amit K, et al. Target organ complications and cardiovascular events associated with masked hypertension and white-coat hypertension: analysis from the Dallas Heart Study. J Am Coll Cardiol. 2016;66:2159–69.
Gkaliagkousi E, Gavriilaki E, Triantafyllou A, Nikolaidou B, Anyfanti P, Koletsos N, et al. Asymmetric dimethylarginine levels are associated with augmentation index across naïve untreated patients with different hypertension phenotypes. J Clin Hypertens. 2018;20:680–5.
Caliskan M, Ciftci O, Gullu H, Caliskan Z, Güven A, Erdogan D, et al. Effect of masked, white-coat, and sustained hypertension on coronary flow reserve and peripheral endothelial functions. Clin Exp Hypertens. 2013;35:183–91.
Wang J, Zhong Y, Ma X, Xiao X, Cheng C, Chen Y, et al. Analyses of endothelial cells and endothelial progenitor cells released microvesicles by using microbead and Q-dot based nanoparticle tracking analysis. Sci Rep. 2016;6:1–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lazaridis, A., Gavriilaki, E., Nikolaidou, B. et al. A study of endothelial and platelet microvesicles across different hypertension phenotypes. J Hum Hypertens 36, 561–569 (2022). https://doi.org/10.1038/s41371-021-00531-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-021-00531-6
This article is cited by
-
Signaling pathways in vascular function and hypertension: molecular mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
Circulating microvesicles across a population with various degree of cardiovascular burden are associated with systolic blood pressure
Journal of Human Hypertension (2023)
-
Assessment of microvesicles from different cell origins in patients with psoriasis: evidence of thrombogenic, proinflammatory microenvironment in the absence of established cardiovascular disease
Journal of Human Hypertension (2022)
-
Differential and targeted vesiculation: pathologic cellular responses to elevated arterial pressure
Molecular and Cellular Biochemistry (2022)