Abstract
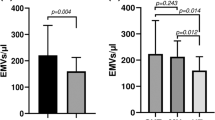
Circulating microvesicles (MVs) have been studied in heterogeneous, divergent, and rather small patient populations with cardiovascular risk . Therefore, we measured endothelial (EMVs), platelet (PMVs) and erythrocyte (RMVs) MVs in patients with divergent cardiovascular risk. We then compared them to coronary artery disease (CAD) and healthy subjects and identified independent MVs’ predictors. We enrolled consecutive patients from our Cardiology, Hypertension, Diabetic, Rheumatic, and Nephrology Outpatient Units with MVs measurements. Central blood pressure (BP) was measured by either applanation tonometry or Mobil-O-graph device, while MVs by a standardized flow cytometry protocol. We studied 369 participants with increased cardiovascular risk: 63 with high cardiovascular risk (47 diabetes mellitus type II/DM and 16 end-stage renal disease/ESRD), 92 with chronic inflammatory disorders and 73 with untreated essential hypertension/UEH. We further included 53 subjects with CAD and 87 otherwise healthy individuals. All MVs were lower in patients with increased cardiovascular risk compared to CAD, showing predictive value with high sensitivity and specificity. Furthermore, PMVs and EMVs were increased in patients with cardiovascular risk compared to healthy individuals. DM and ESRD patients had increased EMVs versus UEH and chronic inflammatory disorders. In the whole study population, RMVs were associated only with history of essential hypertension. In multivariate analysis, systolic BP predicted PMVs. Aage, systolic BP, and DM predicted EMVs. In a large population of patients with divergent cardiovascular risk, MVs are independently associated with systolic blood pressure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data are immediately available upon request from the corresponding author.
References
Organisation WH. Global Status Report on Noncommunicable Diseases 2010. 2011.
Anyfanti P, Gavriilaki E, Douma S, Gkaliagkousi E. Endothelial Dysfunction in Patients with Rheumatoid Arthritis: the Role of Hypertension. Curr Hypertens Rep. 2020;22:56.
Gavriilaki E, Gkaliagkousi E, Sakellari I, Anyfanti P, Douma S, Anagnostopoulos A. Early Prediction of Cardiovascular Risk after Hematopoietic Cell Transplantation: Are We There Yet? Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2019;25:e310–e316.
Gkaliagkousi E, Gavriilaki E, Triantafyllou A, Douma S. Clinical Significance of Endothelial Dysfunction in Essential Hypertension. Curr Hypertens Rep. 2015;17:85.
Ridger VC, Boulanger CM, Angelillo-Scherrer A, Badimon L, Blanc-Brude O, Bochaton-Piallat ML, et al. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb Haemost. 2017;117:1296–316.
Tushuizen ME, Diamant M, Sturk A, Nieuwland R. Cell-derived microparticles in the pathogenesis of cardiovascular disease: friend or foe? Arterioscler, thromb, Vasc Biol. 2011;31:4–9.
Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler, thromb, Vasc Biol. 2011;31:27–33.
Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J, et al. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehab: Off J Eur Soc Cardiol, Working Groups Epidemiol Prev Card Rehab Exerc Physiol. 2011;18:775–89.
Shantsila E, Kamphuisen PW, Lip GY. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis. J Thromb Haemost: JTH. 2010;8:2358–68.
Koga H, Sugiyama S, Kugiyama K, Watanabe K, Fukushima H, Tanaka T, et al. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2005;45:1622–30.
Amabile N, Cheng S, Renard JM, Larson MG, Ghorbani A, McCabe E, et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur Heart J. 2014;35:2972–9.
Chironi G, Simon A, Hugel B, Del Pino M, Gariepy J, Freyssinet JM, et al. Circulating leukocyte-derived microparticles predict subclinical atherosclerosis burden in asymptomatic subjects. Arterioscler, Thromb, Vasc Biol. 2006;26:2775–80.
Montoro-Garcia S, Shantsila E, Wrigley BJ, Tapp LD, Abellan Aleman J, Lip GY. Small-size Microparticles as Indicators of Acute Decompensated State in Ischemic Heart Failure. Rev Esp Cardiol (Engl Ed). 2015;68:951–8.
Gkaliagkousi E, Gavriilaki E, Yiannaki E, Vasileiadis I, Nikolaidou B, Lazaridis A, et al. Platelet microvesicles are associated with the severity of coronary artery disease: comparison between peripheral and coronary circulation. J Thromb Thrombolysis. 2021;51:1138–43.
Gkaliagkousi E, Gavriilaki E, Vasileiadis I, Nikolaidou B, Yiannaki E, Lazaridis A, et al. Endothelial Microvesicles Circulating in Peripheral and Coronary Circulation Are Associated With Central Blood Pressure in Coronary Artery Disease. Am J Hypertens. 2019;32:1199–205.
Gkaliagkousi E, Nikolaidou B, Gavriilaki E, Lazaridis A, Yiannaki E, Anyfanti P, et al. Increased erythrocyte- and platelet-derived microvesicles in newly diagnosed type 2 diabetes mellitus. Diabetes Vasc Dis Res: Off J Int Soc Diabetes Vasc Dis. 2019;16:458–65.
Lazaridis A, Gavriilaki E, Nikolaidou B, Yiannaki E, Dolgyras P, Anyfanti P, et al. A study of endothelial and platelet microvesicles across different hypertension phenotypes. J Hum Hypertens. 2022;36:561–9.
Anyfanti P, Gavriilaki E, Nikolaidou B, Yiannaki E, Lazaridis A, Papadopoulos N, et al. Patients with autoimmune chronic inflammatory diseases present increased biomarkers of thromboinflammation and endothelial dysfunction in the absence of flares and cardiovascular comorbidities. J Thromb Thrombolysis 2022;53:10–6.
Organization WH. Definition and diagnosis of diabetes and intermediate hyperglycemia: report of a WHO/IDF consultation, 2006.
Organization WH. WHO Guidelines Approved by the Guidelines Review Committee. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Abbreviated report of a WHO consultation, 2011.
Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315.
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed). 2017;70:1082.
Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–219.
Gkaliagkousi E, Gavriilaki E, Nikolaidou B, Triantafyllou G, Douma S. Exercise-induced pulse wave velocity changes in untreated patients with essential hypertension: the effect of an angiotensin receptor antagonist. J Clin Hypertens (Greenwich). 2014;16:482–7.
Robert S, Poncelet P, Lacroix R, Arnaud L, Giraudo L, Hauchard A, et al. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost: JTH. 2009;7:190–7.
Bernal-Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, et al. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145:962–70.
Nozaki T, Sugiyama S, Koga H, Sugamura K, Ohba K, Matsuzawa Y, et al. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J Am Coll Cardiol. 2009;54:601–8.
Min PK, Kim JY, Chung KH, Lee BK, Cho M, Lee DL, et al. Local increase in microparticles from the aspirate of culprit coronary arteries in patients with ST-segment elevation myocardial infarction. Atherosclerosis. 2013;227:323–8.
Katopodis JN, Kolodny L, Jy W, Horstman LL, De Marchena EJ, Tao JG, et al. Platelet microparticles and calcium homeostasis in acute coronary ischemias. Am J Hematol. 1997;54:95–101.
Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–3.
Montoro-Garcia S, Shantsila E, Tapp LD, Lopez-Cuenca A, Romero AI, Hernandez-Romero D, et al. Small-size circulating microparticles in acute coronary syndromes: relevance to fibrinolytic status, reparative markers and outcomes. Atherosclerosis. 2013;227:313–22.
Morel O, Hugel B, Jesel L, Mallat Z, Lanza F, Douchet MP, et al. Circulating procoagulant microparticles and soluble GPV in myocardial infarction treated by primary percutaneous transluminal coronary angioplasty. A possible role for GPIIb-IIIa antagonists. J Thromb Haemost: JTH. 2004;2:1118–26.
Zielinska M, Koniarek W, Goch JH, Cebula B, Tybura M, Robak T, et al. Circulating endothelial microparticles in patients with acute myocardial infarction. Kardiologia Pol. 2005;62:531–42.
van der Zee PM, Biro E, Ko Y, de Winter RJ, Hack CE, Sturk A, et al. P-selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin Chem. 2006;52:657–64.
Giannopoulos G, Oudatzis G, Paterakis G, Synetos A, Tampaki E, Bouras G, et al. Red blood cell and platelet microparticles in myocardial infarction patients treated with primary angioplasty. Int J Cardiol. 2014;176:145–50.
Biasucci LM, Porto I, Di Vito L, De Maria GL, Leone AM, Tinelli G, et al. Differences in microparticle release in patients with acute coronary syndrome and stable angina. Circ J : Off J Jpn Circ Soc. 2012;76:2174–82.
Stepien E, Kablak-Ziembicka A, Czyz J, Przewlocki T. Malecki M. Microparticles, not only markers but also a therapeutic target in the early stage of diabetic retinopathy and vascular aging. Expert Opin Therapeutic Targets. 2012;16:677–88.
Zhang Y, Cheng J, Chen F, Wu C, Zhang J, Ren X, et al. Circulating endothelial microparticles and miR-92a in acute myocardial infarction. Biosci Rep. 2017;37:BSR20170047.
Cavallari C, Dellepiane S, Fonsato V, Medica D, Marengo M, Migliori M, et al. Online Hemodiafiltration Inhibits Inflammation-Related Endothelial Dysfunction and Vascular Calcification of Uremic Patients Modulating miR-223 Expression in Plasma Extracellular Vesicles. J Immunol. 2019;202:2372–83.
Burton JO, Hamali HA, Singh R, Abbasian N, Parsons R, Patel AK, et al. Elevated levels of procoagulant plasma microvesicles in dialysis patients. PloS one. 2013;8:e72663.
Trappenburg MC, van Schilfgaarde M, Frerichs FC, Spronk HM, ten Cate H, de Fijter CW, et al. Chronic renal failure is accompanied by endothelial activation and a large increase in microparticle numbers with reduced procoagulant capacity. Nephrol, Dialysis, Transplant: Off Publ Eur Dialysis Transpl Assoc - Eur Ren Assoc. 2012;27:1446–53.
Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol: JASN. 2005;16:3381–8.
Tsimerman G, Roguin A, Bachar A, Melamed E, Brenner B, Aharon A. Involvement of microparticles in diabetic vascular complications. Thromb Haemost. 2011;106:310–21.
Ogata N, Imaizumi M, Nomura S, Shozu A, Arichi M, Matsuoka M, et al. Increased levels of platelet-derived microparticles in patients with diabetic retinopathy. Diabetes Res Clin Pract. 2005;68:193–201.
Feng B, Chen Y, Luo Y, Chen M, Li X, Ni Y. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis. 2010;208:264–9.
Sabatier F, Roux V, Anfosso F, Camoin L, Sampol J, Dignat-George F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood. 2002;99:3962–70.
Anyfanti P, Triantafyllou A, Gkaliagkousi E, Koletsos N, Athanasopoulos G, Zabulis X, et al. Retinal vessel morphology in rheumatoid arthritis: Association with systemic inflammation, subclinical atherosclerosis, and cardiovascular risk. Microcirculation 2017;24. https://doi.org/10.1111/micc.12417.
Anyfanti P, Triantafyllou A, Gkaliagkousi E, Triantafyllou G, Koletsos N, Chatzimichailidou S, et al. Subendocardial viability ratio in patients with rheumatoid arthritis: comparison with healthy controls and identification of prognostic factors. Clin Rheumatol. 2017;36:1229–36.
Anyfanti P, Triantafyllou A, Gkaliagkousi E, Zabulis X, Dolgyras P, Galanopoulou V, et al. Urinary albumin excretion in rheumatoid arthritis is not associated with markers of vasculopathy in distal microvascular beds. Microcirculation. 2019;26:e12514.
Anyfanti P, Gkaliagkousi E, Gavriilaki E, Triantafyllou A, Dolgyras P, Galanopoulou V, et al. Association of galectin-3 with markers of myocardial function, atherosclerosis, and vascular fibrosis in patients with rheumatoid arthritis. Clin Cardiol. 2019;42:62–68.
Anyfanti P, Gkaliagkousi E, Triantafyllou A, Zabulis X, Dolgyras P, Galanopoulou V, et al. Dermal capillary rarefaction as a marker of microvascular damage in patients with rheumatoid arthritis: Association with inflammation and disorders of the macrocirculation. Microcirculation. 2018;25:e12451.
Anyfanti P, Triantafyllou A, Gkaliagkousi E, Koletsos N, Aslanidis S, Douma S. Association of non-invasive hemodynamics with arterial stiffness in rheumatoid arthritis. Scand Cardiovasc. J : SCJ. 2018;52:171–6.
Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–71.
Gotzmann M, Hogeweg M, Seibert FS, Rohn BJ, Bergbauer M, Babel N, et al. Accuracy of fully automated oscillometric central aortic blood pressure measurement techniques. J Hypertens. 2020;38:235–42.
Voukalis C, Shantsila E, Lip GYH. Microparticles and cardiovascular diseases. Ann Med. 2019;51:193–223.
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Shantsila E, Montoro-Garcia S, Gallego P, Lip GY. Circulating microparticles: challenges and perspectives of flow cytometric assessment. Thromb Haemost. 2014;111:1009–14.
Author information
Authors and Affiliations
Contributions
EG designed the study, prepared and edited the manuscript. ElG designed the study, actively participated in subjects’ enrollment and manuscript preparation. AL, BN, and PA analyzed and interpreted the data and participated in manuscript preparations. IV, Am, MEA, and EY actively participated in subject’s enrollment and measurements. AT and DM participated in study design and preparation of tables and figures. IZ, PS, and MD participated in study design and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gavriilaki, E., Lazaridis, A., Anyfanti, P. et al. Circulating microvesicles across a population with various degree of cardiovascular burden are associated with systolic blood pressure. J Hum Hypertens 37, 1105–1111 (2023). https://doi.org/10.1038/s41371-023-00854-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-023-00854-6