Abstract
Background
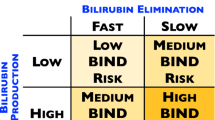
African-American (AA) infants are known to have, overall, lower bilirubin levels than infants of other ethnicities during their birth hospitalization. However, they are known to have a higher incidence of severe hyperbilirubinemia and are over represented in the US Kernicterus Registry. Heme oxygenase-1 (HO) is the rate limiting enzyme in heme metabolism leading to the equimolar production of bilirubin, carbon monoxide (CO) and free iron (Fe). Short (S) (GT)n repeats (<25) in the promoter region of the gene encoding the inducible HO-1 isozyme augment its expression, while long (L) repeats (>33) lead to an attenuation, modulating the production of bilirubin and CO. The impact of HO-1 promoter polymorphisms on bilirubin levels has not been well studied in (AA) infants.
Objective
The objectives of this study were to compare the distribution of (GT)n repeat lengths in the HO-1 promoter region in a cohort of AA infants to those found in other ethnicities and to evaluate the contribution of this polymorphism to the degree of hyperbilirubinemia and the level of COHbc in this cohort.
Methods
We prospectively studied a cohort of term AA infants with O+ mothers. Per hospital routine, infants’ blood type, Rh status, direct antiglobulin test (DAT), and total bilirubin (TB) levels were checked prior to discharge. After parental consent, blood was collected for DNA extraction and carboxyhemoglobin (COHbc) measurements at the same time as the infants’ newborn screen. An infant’s TB percentile risk based on the Bhutani nomogram was used to determine need for phototherapy or follow-up. (GT)n repeat length in the HO-1 promoter was determined for each allele using PCR after DNA extraction from dried bloodspots. Size of allele lengths were typed as short (S, <25), medium (M, 25–33) or long (L, >33).
Results
One hundred eighty infants were studied for a total of 360 separate alleles. 12.2% (44/360) of alleles were S which was significantly less than all other ethnicities reviewed. Carboxyhemoglobin (COHbc) levels and bilirubin percentiles were higher among infants who had at least one S allele when compared to those who had at least one L allele in the cohort as a whole: COHbc 0.92 ± 0.35 vs. 0.85 ± 0.37; p = 0.28 and Bilirubin percentile 48.6 ± 34.0 vs. 44.9 ± 31.6; p = 0.51. This relationship remained when only those infants who were DAT neg were examined: COHbc 0.81 ± 0.26 vs. 0.74 ± 0.21; p = 0.11 and Bilirubin percentile 43.6 ± 29.9 vs. 37.5 ± 28.7; p = 0.28.
Conclusions
The presence of L alleles of this variant is significantly greater among infants who are either African or of African descent. There was a trend toward lower COHbc levels among infants with at least one L allele as opposed to at least one S allele, although this did not have a statistically significant impact on TB risk percentile.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Slusher TM, Zipursky A, Bhutani VK. A global need for affordable neonatal jaundice technologies. Semin Perinatol. 2011;35:185–91.
Stevenson DK, Vreman HJ, Oh W, Fanaroff AA, Wright LL, Lemons JA, et al. Bilirubin production in healthy term infants as measured by carbon monoxide in breath. Clin Chem. 1994;40:1934–9.
Kaplan M, Muraca M, Hammerman C, Rubaltelli FF, Vilei MT, Vreman HJ, et al. Imbalance between production and conjugation of bilirubin: a fundamental concept in the mechanism of neonatal jaundice. Pediatrics. 2002;110:e47.
Watchko JF, Lin Z. Exploring the genetic architecture of neonatal hyperbilirubinemia. Semin Fetal Neonatal Med. 2010;15:168–75.
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316.
Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term infants. Pediatrics. 1999;13:6–14.
Kaplan M, Wong RJ, Stevenson DK. Heme oxygenase-1 promoter polymorphisms: do they modulate neonatal hyperbilirubinemia? J Perinatol. 2017;37:901–5.
Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–104.
Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, et al. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66:187–95.
Chou SC, Palmer RH, Ezhuthachan S, Newman C, Pradell-Boyd B, Maisels MJ, et al. Management of hyperbilirubinemia in newborns: measuring performance by using a benchmarking model. Pediatrics. 2003;112:1264–73.
Newman TB, Escobar GJ, Gonzales VM, Armstrong MA, Gardner MN, Folck BF. Frequency of bilirubin testing and hyperbilirubinemia in a large health maintenance organization. Pediatrics. 1999;104:198–203.
Johnson L, Bhutani VK, Karp K, Sivieri EM, Shapiro SM. Clinical report from the pilot USA kernicterus registry (1992-2004). J Perinatol. 2009;29:S25–S45.
Watchko JF. Hyperbilirubinemia in African American neonates: clinical issues and current challenges. Semin Fetal Neonatal Med. 2010;15:176–82.
Schutzman DL, Gatien E, Ajayi S, Wong RJ. Carboxyhemoglobin levels as a predictor of risk for significant hyperbilirubinemia in African-American DAT + infants. J Perinatol. 2016;36:386–8.
St Julien KR, Jellife-Pawlowski LL, Shaw GM, Stevenson DK, O’Bradovich HM, Krasnow MA, Stanford BPD Study Group. High quality genome-wide genotyping from archived dried blood spots without DNA amplification. PLoS ONE. 2013;8:e64710.
Stevenson DK, Fanaroff AA, Maisels MJ, Young BWY, Wong RJ, Vreman HJ, et al. Prediction of hyperbilirubinemia in near-term and term infants. Pediatrics. 2001;108:31–9.
Keren R, Luan X, Friedman S, Saddlemire S, Cnaan A, Bhutani VK. A comparison of alternative risk assessment strategies for predicting significant hyperbilirubinemia in term and near term infants. Pediatrics. 2008;121:e170–9.
Wickremasinghe AC, Kuzniewicz MW, Newman TB. Black race is not protective against hazardous bilirubin levels. J Pediatr. 2013;162:1068–9.
Chen YH, Chau LY, Chen JW, Lin SJ. Serum bilirubin and ferritin levels link heme oxygenase-1 gene promotor polymorphism and susceptibility to coronary artery disease in diabetic patients. Diabetes Care. 2008;31:1615–20.
Endler G, Exner M, Schillinger M, Marculescu R, Sunder-Plassmann R, Raith M, et al. A microsatellite polymorphism in the heme oxygenase-1 promoter is associated with increased bilirubin and HDL levels but not with coronary artery disease. Thromb Haemost. 2004;91:155–61.
Zhou Y, Wang SN, Li H, Zha W, Peng Q, Li S, et al. Quantitative trait analysis of polymorphisms in two bilirubin metabolism enzymes to physiologic bilirubin levels in Chinese newborns. J Pediatr. 2014;165:1154–60.
Bozkaya OG, Kumral A, Yesilirmak DC, Ulgenalp A, Duman N, Ercal D, Ozkan H. Prolonged unconjugated hyperbilirubinemia associated with the haem oxygenase-1 gene promoter polymorphism. Acta Paediatr. 2010;99:679–83.
Schutzman DL, Baudhuin LM, Gatien E, Ajayi S, Wong RJ. Effect of genetic variants of bilirubin metabolism on the degree of hyperbilirubinemia in African-American newborns. J Perinatol. 2017;37:432–5.
Hansson HH, Maretty L, Balle C, Goka BQ, Luzon E, Nkrumah FN, et al. Polymorphisms in haem oxygenase-1 promoter are not associated with severity of plasmodium falciparum malaria in Ghanaian children. Malar J. 2015;14:153.
Shibahara S, Kita muro T, Takahashi K. Heme degredation and human disease: diversity is the soul of life. Antioxid Redox Signal. 2002;4:593–602.
Sambo MR, Trovoada MJ, Benchimol C, Quinhentos V, Goncalves L, Velosa R, et al. Transforming growth factor beta 2 and haem oxygenase-1 genes are risk factors for the cerebral malaria syndrome in Angolan children. PLoS ONE. 2010;5:e11141.
Walther M, De CA, Aka P, Njie M, Amambua-Ngwa A, Walther B, et al. HMOX1 gene promoter alleles and high HO-1 levels are associated with severe malaria in Gambian children. PLoS Pathog. 2012;8:e1002579.
Tiwari PK, Sethi A, Basu S, Raman R, Kumar A. Heme oxygenase-1 gene variants and hyperbilirubinemia risk in North Indian infants. Eur J Pediatr. 2013;172:1627–32.
Kaplan M, Renbaum P, Hammerman C, Vreman HJ, Wong RJ, Stevenson DK. Heme oxygenase-1 promoter polymorphisms and neonatal jaundice. Neonatology. 2014;106:323–9.
Kanai M, Akaba K, Sasaki A, Sato M, Harano T, Shibahara S, et al. Neonatal hyperbilirubinemia in Japanese neonates: analysis of the heme oxygenase-1 gene and fetal hemoglobin concentration in cord blood. Pediatr Res. 2003;54:165–71.
Acknowledgements
The authors would like to acknowledge Flora Kalish for performing the DNA extractions and sequencing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Schutzman, D.L., Gatien, E., Ajayi, S. et al. Heme oxygenase-1 genetic variants and the conundrum of hyperbilirubinemia in African-American newborns. J Perinatol 38, 345–350 (2018). https://doi.org/10.1038/s41372-017-0039-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-017-0039-x