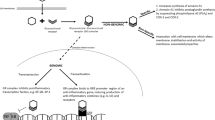
Abstract
Mechanical ventilation is necessary to maintain oxygenation and ventilation in many preterm infants. Unfortunately, even short periods of mechanical ventilation can cause lung and airway injury, and initiate the lung inflammation that contributes to the development of bronchopulmonary dysplasia (BPD). The mechanical stretch leads to airway cell differentiation and simplification of the alveoli, and releases cytokines that cause systemic response in other organs. Mechanical ventilation also leads to brain injury (IVH, white and gray matter) and neuronal inflammation that can affect the neurodevelopment of preterm infants. In efforts to decrease BPD, corticosteroids have been used for both prevention and treatment of lung inflammation. Corticosteroids have also been demonstrated to cause neuronal injury, so the clinician must balance the negative effects of both mechanical ventilation and steroids on the brain and lungs. Predictive models for BPD can help assess the infants who will benefit most from corticosteroid exposure. This review describes the lung and brain injury from mechanical ventilation in the delivery room and chronic mechanical ventilation in animal models. It provides updates on the current guidelines for use of postnatal corticosteroids (dexamethasone, hydrocortisone, budesonide, budesonide with surfactant) for the prevention and treatment of BPD, and the effects the timing of each steroid regimen has on neurodevelopment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Horbar JD, Edwards EM, Greenberg LT, Morrow KA, Soll RF, Buus-Frank ME, et al. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatr. 2017;171:e164396.
Jensen EA, Edwards EM, Greenberg LT, Soll RF, Ehret DEY, Horbar JD. Severity of bronchopulmonary dysplasia among very preterm infants in the United States. Pediatrics. 2021;148:e2020030007.
Hillman NH, Moss TJ, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, et al. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med. 2007;176:575–81.
Hillman NH, Moss TJ, Nitsos I, Jobe AH. Moderate tidal volumes and oxygen exposure during initiation of ventilation in preterm fetal sheep. Pediatr Res. 2012;72:593–9.
Hillman NH, Polglase GR, Pillow JJ, Saito M, Kallapur SG, Jobe AH. Inflammation and lung maturation from stretch injury in preterm fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2011;300:L232–241.
Thebaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, et al. Bronchopulmonary dysplasia. Nat Rev Dis Prim. 2019;5:78.
Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA. 2016;316:611–24.
Polglase GR, Miller SL, Barton SK, Baburamani AA, Wong FY, Aridas JD, et al. Initiation of resuscitation with high tidal volumes causes cerebral hemodynamic disturbance, brain inflammation and injury in preterm lambs. PLoS One. 2012;7:e39535.
Cheong JLY, Doyle LW. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin Perinatol. 2018;42:478–84.
Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. J Pediatr. 2014;165:1258–60.
Jobe AH, Hillman N, Polglase G, Kramer BW, Kallapur S, Pillow J. Injury and inflammation from resuscitation of the preterm infant. Neonatology. 2008;94:190–6.
Deptula N, Royse E, Kemp MW, Miura Y, Kallapur SG, Jobe AH, et al. Brief mechanical ventilation causes differential epithelial repair along the airways of fetal, preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2016;311:L412–420.
Resende JG, Menezes CG, Paula AM, Ferreira AC, Zaconeta CA, Silva CA, et al. Evaluation of peak inspiratory pressure and respiratory rate during ventilation of an infant lung model with a self-inflating bag. J Pediatr (Rio J). 2006;82:359–64.
Bjorland PA, Ersdal HL, Haynes J, Ushakova A, Oymar K, Rettedal SI. Tidal volumes and pressures delivered by the NeoPuff T-piece resuscitator during resuscitation of term newborns. Resuscitation. 2022;170:222–9.
Tingay DG, Pereira-Fantini PM, Oakley R, McCall KE, Perkins EJ, Miedema M, et al. Gradual aeration at birth is more lung protective than a sustained inflation in preterm lambs. Am J Respir Crit Care Med. 2019;200:608–16.
Hillman NH, Nitsos I, Berry C, Pillow JJ, Kallapur SG, Jobe AH. Positive end-expiratory pressure and surfactant decrease lung injury during initiation of ventilation in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2011;301:L712–720.
Hooper SB, Te Pas AB, Kitchen MJ. Respiratory transition in the newborn: a three-phase process. Arch Dis Child Fetal Neonatal Ed. 2016;101:F266–271.
Barton SK, Moss TJ, Hooper SB, Crossley KJ, Gill AW, Kluckow M, et al. Protective ventilation of preterm lambs exposed to acute chorioamnionitis does not reduce ventilation-induced lung or brain injury. PLoS One. 2014;9:e112402.
Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–64.
Hernandez LA, Peevy KJ, Moise AA, Parker JC. Chest wall restriction limits high airway pressure-induced lung injury in young rabbits. J Appl Physiol. 1989;66:2364–8.
Taskar V, John J, Evander E, Robertson B, Jonson B. Surfactant dysfunction makes lungs vulnerable to repetitive collapse and reexpansion. Am J Respir Crit Care Med. 1997;155:313–20.
Keszler M. Mechanical ventilation strategies. Semin Fetal Neonatal Med. 2017;22:267–74.
Hillman NH, Kemp MW, Miura Y, Kallapur SG, Jobe AH. Sustained inflation at birth did not alter lung injury from mechanical ventilation in surfactant-treated fetal lambs. PLoS One. 2014;9:e113473.
Kapadia VS, Urlesberger B, Soraisham A, Liley HG, Schmolzer GM, Rabi Y, et al. Sustained lung inflations during neonatal resuscitation at birth: a meta-analysis. Pediatrics. 2021;147:e2020021204.
Bamat N, Fierro J, Wang Y, Millar D, Kirpalani H. Positive end-expiratory pressure for preterm infants requiring conventional mechanical ventilation for respiratory distress syndrome or bronchopulmonary dysplasia. Cochrane Database Syst Rev. 2019;2:CD004500.
Sandhar BK, Niblett DJ, Argiras EP, Dunnill MS, Sykes MK. Effects of positive end-expiratory pressure on hyaline membrane formation in a rabbit model of the neonatal respiratory distress syndrome. Intensive Care Med. 1988;14:538–46.
Robertson B, Berry D, Curstedt T, Grossmann G, Ikegami M, Jacobs H, et al. Leakage of protein in the immature rabbit lung; effect of surfactant replacement. Respir Physiol. 1985;61:265–76.
Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–38.
Hughes KT, Beasley MB. Pulmonary manifestations of acute lung injury: more than just diffuse alveolar damage. Arch Pathol Lab Med. 2017;141:916–22.
Hillman NH, Kallapur SG, Pillow JJ, Moss TJ, Polglase GR, Nitsos I, et al. Airway injury from initiating ventilation in preterm sheep. Pediatr Res. 2010;67:60–65.
Abugisisa L, Royse EX, Kemp MW, Jobe AH, Hillman NH. Preterm ovine respiratory epithelial cell responses to mechanical ventilation, lipopolysaccharide, and interleukin 13. Am J Physiol Lung Cell Mol Physiol. 2023. https://doi.org/10.1152/ajplung.00355.2022. Online ahead of print.
Dai H, Pan L, Lin F, Ge W, Li W, He S. Mechanical ventilation modulates Toll-like receptors 2, 4, and 9 on alveolar macrophages in a ventilator-induced lung injury model. J Thorac Dis. 2015;7:616–24.
Jobe AH, Ikegami M. Lung development and function in preterm infants in the surfactant treatment era. Annu Rev Physiol. 2000;62:825–46.
Hennessy EM, Bracewell M, Wood N, Wolke D, Costeloe KL, Gibson AT, et al. Respiratory health in pre-school and school age children following extremely preterm birth. Arch Dis Child. 2008;93:1037–43.
Polglase GR, Hillman NH, Ball MK, Kramer BW, Kallapur SG, Jobe AH, et al. Title: lung and systemic inflammation in preterm lambs on CPAP or conventional ventilation. Pediatr Res. 2009;65:67–71.
Schmolzer GM, Kumar M, Pichler G, Aziz K, O’Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ-Brit Med J. 2013;347:f5980.
Mayer CA, Ganguly A, Mayer A, Pabelick CM, Prakash YS, Hascall VC, et al. CPAP-induced airway hyper-reactivity in mice is modulated by hyaluronan synthase-3. Pediatr Res. 2022;92:685–93.
Albertine KH. Progress in understanding the pathogenesis of BPD using the baboon and sheep models. Semin Perinatol. 2013;37:60–68.
Surate Solaligue DE, Rodriguez-Castillo JA, Ahlbrecht K, Morty RE. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2017;313:L1101–L1153.
Brew N, Hooper SB, Zahra V, Wallace M, Harding R. Mechanical ventilation injury and repair in extremely and very preterm lungs. PLoS One. 2013;8:e63905.
Narayanan M, Beardsmore CS, Owers-Bradley J, Dogaru CM, Mada M, Ball I, et al. Catch-up alveolarization in ex-preterm children: evidence from (3)He magnetic resonance. Am J Respir Crit Care Med. 2013;187:1104–9.
Cannavo L, Perrone S, Viola V, Marseglia L, Di Rosa G, Gitto E. Oxidative stress and respiratory diseases in preterm newborns. Int J Mol Sci. 2021;22:12504.
Kalikkot Thekkeveedu R, El-Saie A, Prakash V, Katakam L, Shivanna B. Ventilation-induced lung injury (VILI) in neonates: evidence-based concepts and lung-protective strategies. J Clin Med. 2022;11:557.
Hillman NH, Kallapur SG, Pillow JJ, Nitsos I, Polglase GR, Ikegami M, et al. Inhibitors of inflammation and endogenous surfactant pool size as modulators of lung injury with initiation of ventilation in preterm sheep. Respir Res. 2010;11:151.
Ball MK, Jobe AH, Polglase GR, Kallapur SG, Cheah FC, Hillman NH, et al. High and low body temperature during the initiation of ventilation for near-term lambs. Resuscitation. 2009;80:133–7.
Mulrooney N, Champion Z, Moss TJ, Nitsos I, Ikegami M, Jobe AH. Surfactant and physiological responses of preterm lambs to continuous positive airway pressure. Am J Respir Crit Care Med. 2005;171:1–6.
Brumley GW, Hodson WA, Avery ME. Lung phospholipids and surface tension correlations in infants with and without hyaline membrane disease and in adults. Pediatr. 1967;40:13–19.
McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12:CD004454.
Hillman NH, Pillow JJ, Ball MK, Polglase GR, Kallapur SG, Jobe AH. Antenatal and postnatal corticosteroid and resuscitation induced lung injury in preterm sheep. Respir Res. 2009;10:124.
Abiramalatha T, Ramaswamy VV, Bandyopadhyay T, Somanath SH, Shaik NB, Pullattayil AK, et al. Interventions to prevent bronchopulmonary dysplasia in preterm neonates: an umbrella review of systematic reviews and meta-analyses. JAMA Pediatr. 2022;176:502–16.
Abdel-Latif ME, Davis PG, Wheeler KI, De Paoli AG, Dargaville PA. Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev. 2021;5:CD011672.
Devi U, Roberts KD, Pandita A. A systematic review of surfactant delivery via laryngeal mask airway, pharyngeal instillation, and aerosolization: methods, limitations, and outcomes. Pediatr Pulmonol. 2022;57:9–19.
Ng EH, Shah V. Guidelines for surfactant replacement therapy in neonates. Paediatr Child Health. 2021;26:35–49.
Peng W, Zhu H, Shi H, Liu E. Volume-targeted ventilation is more suitable than pressure-limited ventilation for preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2014;99:F158–165.
Ackermann BW, Klotz D, Hentschel R, Thome UH, van Kaam AH. High-frequency ventilation in preterm infants and neonates. Pediatr Res. 2022. https://doi.org/10.1038/s41390-021-01639-8. Online ahead of print.
Albertine KH. Brain injury in chronically ventilated preterm neonates: collateral damage related to ventilation strategy. Clin Perinatol. 2012;39:727–40.
Chan KYY, Miller SL, Schmolzer GM, Stojanovska V, Polglase GR. Respiratory support of the preterm neonate: lessons about ventilation-induced brain injury from large animal models. Front Neurol. 2020;11:862.
Mian Q, Cheung PY, O’Reilly M, Barton SK, Polglase GR, Schmolzer GM. Impact of delivered tidal volume on the occurrence of intraventricular haemorrhage in preterm infants during positive pressure ventilation in the delivery room. Arch Dis Child Fetal Neonatal Ed. 2019;104:F57–F62.
Alahmari DM, Chan KYY, Stojanovska V, LaRosa D, Barton SK, Nitsos I, et al. Diffusion tensor imaging detects ventilation-induced brain injury in preterm lambs. PLoS One. 2017;12:e0188737.
Hillman NH, Kothe TB, Schmidt AF, Kemp MW, Royse E, Fee E, et al. Surfactant plus budesonide decreases lung and systemic responses to injurious ventilation in preterm sheep. Am J Physiol Lung Cell Mol Physiol. 2020;318:L41–8.
Loeliger M, Inder T, Cain S, Ramesh RC, Camm E, Thomson MA, et al. Cerebral outcomes in a preterm baboon model of early versus delayed nasal continuous positive airway pressure. Pediatrics. 2006;118:1640–53.
Patra A, Huang H, Bauer JA, Giannone PJ. Neurological consequences of systemic inflammation in the premature neonate. Neural Regen Res. 2017;12:890–6.
Brouwer MJ, Kersbergen KJ, van Kooij BJM, Benders M, van Haastert IC, Koopman-Esseboom C, et al. Preterm brain injury on term-equivalent age MRI in relation to perinatal factors and neurodevelopmental outcome at two years. PLoS One. 2017;12:e0177128.
Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–161.
Leviton A, Kuban K, O’Shea TM, Paneth N, Fichorova R, Allred EN, et al. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J Pediatr. 2011;158:897–903.
Malaeb SN, Stonestreet BS. Steroids and injury to the developing brain: net harm or net benefit? Clin Perinatol. 2014;41:191–208.
Htun ZT, Schulz EV, Desai RK, Marasch JL, McPherson CC, Mastrandrea LD, et al. Postnatal steroid management in preterm infants with evolving bronchopulmonary dysplasia. J Perinatol. 2021;41:1783–96.
Vinukonda G, Dummula K, Malik S, Hu F, Thompson CI, Csiszar A, et al. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke. 2010;41:1766–73.
Meijer OC, de Lange EC, Breimer DD, de Boer AG, Workel JO, de Kloet ER. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology. 1998;139:1789–93.
Crochemore C, Lu J, Wu Y, Liposits Z, Sousa N, Holsboer F, et al. Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry. 2005;10:790–8.
Grossmann C, Scholz T, Rochel M, Bumke-Vogt C, Oelkers W, Pfeiffer AF, et al. Transactivation via the human glucocorticoid and mineralocorticoid receptor by therapeutically used steroids in CV-1 cells: a comparison of their glucocorticoid and mineralocorticoid properties. Eur J Endocrinol. 2004;151:397–406.
Doyle LW. Postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Neonatology. 2021;118:244–51.
Cummings JJ. PAACOFAN. Postnatal corticosteroids to prevent or treat chronic lung disease following preterm birth. Pediatrics. 2022;149:e2022057530.
Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Late (>/= 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2021;11:CD001145.
Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Early (<7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2021;10:CD001146.
Parikh NA, Lasky RE, Kennedy KA, Moya FR, Hochhauser L, Romo S, et al. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics. 2007;119:265–72.
Murphy BP, Inder TE, Huppi PS, Warfield S, Zientara GP, Kikinis R, et al. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics. 2001;107:217–21.
Ramaswamy VV, Bandyopadhyay T, Nanda D, Bandiya P, Ahmed J, Garg A, et al. Assessment of postnatal corticosteroids for the prevention of bronchopulmonary dysplasia in preterm neonates: a systematic review and network meta-analysis. JAMA Pediatr. 2021;175:e206826.
Puia-Dumitrescu M, Wood TR, Comstock BA, Law JB, German K, Perez KM, et al. Dexamethasone, prednisolone, and methylprednisolone use and 2-year neurodevelopmental outcomes in extremely preterm infants. JAMA Netw Open. 2022;5:e221947.
Scott SM, Watterberg KL. Effect of gestational age, postnatal age, and illness on plasma cortisol concentrations in premature infants. Pediatr Res. 1995;37:112–6.
Baud O, Watterberg KL. Prophylactic postnatal corticosteroids: early hydrocortisone. Semin Fetal Neonatal Med. 2019;24:202–6.
Shaffer ML, Baud O, Lacaze-Masmonteil T, Peltoniemi OM, Bonsante F, Watterberg KL. Effect of prophylaxis for early adrenal insufficiency using low-dose hydrocortisone in very preterm infants: an individual patient data meta-analysis. J Pediatr. 2019;207:136–42.
Kersbergen KJ, de Vries LS, van Kooij BJ, Isgum I, Rademaker KJ, van Bel F, et al. Hydrocortisone treatment for bronchopulmonary dysplasia and brain volumes in preterm infants. J Pediatr. 2013;163:666–71.
Alison M, Tilea B, Toumazi A, Biran V, Mohamed D, Alberti C, et al. Prophylactic hydrocortisone in extremely preterm infants and brain MRI abnormality. Arch Dis Child Fetal Neonatal Ed. 2020;105:520–5.
Onland W, Cools F, Kroon A, Rademaker K, Merkus MP, Dijk PH, et al. Effect of hydrocortisone therapy initiated 7 to 14 days after birth on mortality or bronchopulmonary dysplasia among very preterm infants receiving mechanical ventilation: a randomized clinical trial. JAMA. 2019;321:354–63.
Watterberg KL, Walsh MC, Li L, Chawla S, D’Angio CT, Goldberg RN, et al. Hydrocortisone to improve survival without bronchopulmonary dysplasia. N. Engl J Med. 2022;386:1121–31.
Halbmeijer NM, Onland W, Cools F, Swarte R, van der Heide-Jalving M, Merkus MP, et al. Effect of systemic hydrocortisone initiated 7 to 14 days after birth in ventilated preterm infants on mortality and neurodevelopment at 2 years’ corrected age: follow-up of a randomized clinical trial. JAMA. 2021;326:355–7.
Zeng L, Tian J, Song F, Li W, Jiang L, Gui G, et al. Corticosteroids for the prevention of bronchopulmonary dysplasia in preterm infants: a network meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2018;103:F506–F511.
Bhandari A, Schramm CM, Kimble C, Pappagallo M, Hussain N. Effect of a short course of prednisolone in infants with oxygen-dependent bronchopulmonary dysplasia. Pediatrics. 2008;121:e344–9.
Linafelter A, Cuna A, Liu C, Quigley A, Truog WE, Sampath V, et al. Extended course of prednisolone in infants with severe bronchopulmonary dysplasia. Early Hum Dev. 2019;136:1–6.
Allen DB. Inhaled corticosteroids and endocrine effects in childhood. Endocrinol Metab Clin North Am. 2020;49:651–65.
Moore CD, Roberts JK, Orton CR, Murai T, Fidler TP, Reilly CA, et al. Metabolic pathways of inhaled glucocorticoids by the CYP3A enzymes. Drug Metab Dispos. 2013;41:379–89.
Bassler D, Shinwell ES, Hallman M, Jarreau PH, Plavka R, Carnielli V, et al. Long-term effects of inhaled budesonide for bronchopulmonary dysplasia. N. Engl J Med. 2018;378:148–57.
Onland W, Offringa M, van Kaam A. Late (>/= 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;8:CD002311.
Chen CM, Chang CH, Chao CH, Wang MH, Yeh TF. Biophysical and chemical stability of surfactant/budesonide and the pulmonary distribution following intra-tracheal administration. Drug Deliv. 2019;26:604–11.
Kothe TB, Kemp MW, Schmidt AF, Royse E, Salomone F, Clarke MW, et al. Surfactant plus budesonide decreases lung and systemic inflammation in mechanically ventilated preterm sheep. Am J Physiol Lung Cell Mol Physiol. 2019;316:L888–L893.
Yeh TF, Chen CM, Wu SY, Husan Z, Li TC, Hsieh WS, et al. Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2016;193:86–95.
Yeh TF, Lin HC, Chang CH, Wu TS, Su BH, Li TC, et al. Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: a pilot study. Pediatrics. 2008;121:e1310–1318.
Kothe TB, Sadiq FH, Burleyson N, Williams HL, Anderson C, Hillman NH. Surfactant and budesonide for respiratory distress syndrome: an observational study. Pediatr Res. 2020;87:940–5.
Hillman NH, Abugisisa L, Royse E, Fee E, Kemp MW, Kramer BW, et al. Dose of budesonide with surfactant affects lung and systemic inflammation after normal and injurious ventilation in preterm lambs. Pediatr Res. 2020;88:726–32.
Becker SA, Kothe TB, Josephsen JB, Jackson K, Williams HL, Hillman NH. Early physiological and adrenal effects of budesonide mixed with surfactant in large observational preterm cohort study. Neonatology. 2022;119:474–82.
Anderson CD, Kothe TB, Josephsen JB, Sadiq FH, Burleyson N, Williams HL, et al. Budesonide mixed with surfactant did not affect neurodevelopmental outcomes at 6 or 18 months corrected age in observational cohorts. J Perinatol. 2021;41:1681–9.
Kuo HT, Lin HC, Tsai CH, Chouc IC, Yeh TF. A follow-up study of preterm infants given budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants. J Pediatr. 2010;156:537–41.
McEvoy CT, Ballard PL, Ward RM, Rower JE, Wadhawan R, Hudak ML, et al. Dose-escalation trial of budesonide in surfactant for prevention of bronchopulmonary dysplasia in extremely low gestational age high-risk newborns (SASSIE). Pediatr Res. 2020;88:629–36.
Ballard PL, Torgerson D, Wadhawan R, Hudak ML, Weitkamp JH, Harris J, et al. Blood metabolomics in infants enrolled in a dose escalation pilot trial of budesonide in surfactant. Pediatr Res. 2021;90:784–94.
Hillman NH, Kemp MW, Fee E, Rittenschober-Bohm J, Royse E, Abugisisa L, et al. Budesonide with surfactant decreases systemic responses in mechanically ventilated preterm lambs exposed to fetal intra-amniotic lipopolysaccharide. Pediatr Res. 2021;90:328–34.
Funding
This work was supported by NIH grants R21- HD100721-01 (NHH).
Author information
Authors and Affiliations
Contributions
NHH drafted, revised, and approved the manuscript. AJH revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hillman, N.H., Jobe, A.H. Preterm lung and brain responses to mechanical ventilation and corticosteroids. J Perinatol 43, 1222–1229 (2023). https://doi.org/10.1038/s41372-023-01692-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01692-7