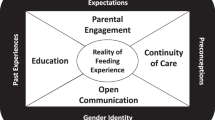
Abstract
Introduction
Parents struggle with being asked to participate in neonatal research. Past work has largely failed to include views of minoritized parents, low-socioeconomic status parents, and those who declined research. We aimed to describe parents’ preferences related to learning about eligibility for neonatal research.
Methods
Qualitative interviews of parents who were asked to enroll their infant in neonatal research. Themes related to parental experiences and preferences for learning about neonatal research were identified using content analysis.
Results
Many parents desired greater involvement of their clinical team. Emotions at the time of recruitment were critically important to parents’ experience, where were deeply impacted by interpersonal relationships with research staff.
Discussion
Increased involvement of the clinical team and greater sensitivity to the stressors around parent and infant conditions at the time of recruitment for neonatal research should be considered by those attempting to improve recruitment for neonatal research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
Data that support the findings of this study are available on request from the corresponding author (EMW). The data are not publicly available due to restrictions on information that could compromise the privacy of research participants. Depending on the nature of the request, approval from the Seattle Children’s Hospital IRB may be required.
References
Nordheim T, Anderzén-Carlsson A, Nakstad B. A qualitative study of the experiences of Norwegian parents of very low birthweight infants enrolled in a randomized nutritional trial. J Pediatr Nurs. 2018;43:e66–74. https://doi.org/10.1016/j.pedn.2018.07.008.
Al Maghaireh DF, Abdullah KL, Chan CM, Piaw CY, Al Kawafha MM. Systematic review of qualitative studies exploring parental experiences in the Neonatal Intensive Care Unit. J Clin Nurs. 2016;25:2745–56. https://doi.org/10.1111/jocn.13259.
Rich WD, Auten KJ, Gantz MG, Hale EC, Hensman AM, Newman NS, et al. Antenatal consent in the SUPPORT trial: challenges, costs, and representative enrollment. Pediatrics. 2010;126:e215–21. https://doi.org/10.1542/peds.2009-3353.
Levett KM, Roberts CL, Simpson JM, Morris JM. Site-specific predictors of successful recruitment to a perinatal clinical trial. Clin Trials. 2014;11:584–9. https://doi.org/10.1177/1740774514543539.
Guttmann KF, Li S, Wu YW, Juul SE, Wilfond BS, Weiss EM, et al. Factors that impact hospital-specific enrollment rates for a neonatal clinical trial: an analysis of the HEAL study. Ethics Hum Res. 2023;45:29–38. https://doi.org/10.1002/eahr.500154.
Paskett ED, Reeves KW, McLaughlin JM, Katz ML, McAlearney AS, Ruffin MT, et al. Recruitment of minority and underserved populations in the United States: the Centers for Population Health and Health Disparities experience. Contemp Clin Trials. 2008;29:847–61. https://doi.org/10.1016/j.cct.2008.07.006.
Kelly ML, Ackerman PD, Ross LF. The participation of minorities in published pediatric research. J Natl Med Assoc. 2005;97:777–83.
Walsh C, Ross LF. Are minority children under- or overrepresented in pediatric research? Pediatrics. 2003;112:890–5. https://doi.org/10.1542/peds.112.4.890.
Natale JE, Lebet R, Joseph JG, Ulysse C, Ascenzi J, Wypij D, et al. Racial and ethnic disparities in parental refusal of consent in a large, multisite pediatric critical care clinical trial. J Pediatr 2017;184:204–208.e1. https://doi.org/10.1016/j.jpeds.2017.02.006.
Paquette E, Shukla A, Duyar S. Social determinants of research engagement and implications for precision medicine: sociodemographic factors associated with differential Enrollment in a pediatric critical care biorepository. Toronto, ON, Canada: Pediatric Academic Societies; 2018.
Liu L, Krailo M, Reaman GH, Bernstein L, Surveillance EiaERCCLG. Childhood cancer patients’ access to cooperative group cancer programs: a population-based study. Cancer. 2003;97:1339–45. https://doi.org/10.1002/cncr.11192.
Weiss EM, Olszewski AE, Guttmann KF, Magnus BE, Li S, Shah AR, et al. Parental factors associated with the decision to participate in a neonatal clinical trial. JAMA Netw Open. 2021;4:e2032106. https://doi.org/10.1001/jamanetworkopen.2020.32106.
Foglia EE, Nolen TL, DeMauro SB, Das A, Bell EF, Stoll BJ, et al. Short-term outcomes of infants enrolled in randomized clinical trials vs those eligible but not enrolled. JAMA. 2015;313:2377–9. https://doi.org/10.1001/jama.2015.5734.
Anderson JG, Rogers EE, Baer RJ, Oltman SP, Paynter R, Partridge JC, et al. Racial and ethnic disparities in preterm infant mortality and severe morbidity: a population-based study. Neonatology. 2018;113:44–54. https://doi.org/10.1159/000480536.
Wallace ME, Mendola P, Kim SS, Epps N, Chen Z, Smarr M, et al. Racial/ethnic differences in preterm perinatal outcomes. Am J Obstet Gynecol. 2017;216:306.e1–12. https://doi.org/10.1016/j.ajog.2016.11.1026.
Beck AF, Edwards EM, Horbar JD, Howell EA, McCormick MC, Pursley DM. The color of health: how racism, segregation, and inequality affect the health and well-being of preterm infants and their families. Pediatr Res. 2020;87:227–34. https://doi.org/10.1038/s41390-019-0513-6.
Janevic T, Zeitlin J, Auger N, Egorova NN, Hebert P, Balbierz A, et al. Association of race/ethnicity with very preterm neonatal morbidities. JAMA Pediatr. 2018;172:1061–9. https://doi.org/10.1001/jamapediatrics.2018.2029.
Weiss EM, Guttmann KF, Olszewski AE, Magnus BE, Li S, Kim SYH, et al. Parental enrollment decision-making for a neonatal clinical trial. J Pediatr. 2021;239:143–149.e3. https://doi.org/10.1016/j.jpeds.2021.08.014.
Shah AR, Wilfond BS, Silvia A, Hancuch K, Woodrum D, Heagerty P, et al. Informed consent for a neonatal clinical trial: parental experiences and perspectives. J Perinatol. 2018;38:865–72. https://doi.org/10.1038/s41372-018-0119-6.
Zupancic JA, Gillie P, Streiner DL, Watts JL, Schmidt B. Determinants of parental authorization for involvement of newborn infants in clinical trials. Pediatrics. 1997;99:e6.
Gillies K, Elwyn G, Cook J. Making a decision about trial participation: the feasibility of measuring deliberation during the informed consent process for clinical trials. Trials. 2014;15:307. https://doi.org/10.1186/1745-6215-15-307.
Dahan S, Jung C, Dassieu G, Durrmeyer X, Caeymaex L. Trust and consent: a prospective study on parents’ perspective during a neonatal trial. J Med Ethics. 2021;47:678–83.
Nathe JM, Oskoui TT, Weiss EM. Parental views of facilitators and barriers to research participation: systematic review. Pediatrics. 2023;151. https://doi.org/10.1542/peds.2022-058067.
Charmaz K. Constructing grounded theory. Sage Publications; London; 2006.
Sotto-Santiago S. Time to reconsider the word minority in academic medicine. J Best Pr Health Prof Divers. 2019;12:72–8.
Wingrove-Haugland E, McLeod J. Not “minority” but “minoritized”. Teach Ethics. 2021;21:1–11.
Armstrong K, Ritchie C. Research participation in marginalized communities—overcoming barriers. N Engl J Med. 2022;386:203–5. https://doi.org/10.1056/NEJMp2115621.
Benton TD. Suicide and suicidal behaviors among minoritized youth. Child Adolesc Psychiatr Clin N Am. 2022;31:211–21. https://doi.org/10.1016/j.chc.2022.01.002.
Barry D, Steinberg JR, Towner M, Barber EL, Simon MA, Roque DR. Enrollment of racial and ethnic minoritized groups in gynecologic oncology clinical trials: a review of the scope of the problem, contributing factors, and strategies to improve inclusion. Clin Obstet Gynecol. 2023;66:22–35. https://doi.org/10.1097/GRF.0000000000000765.
Njoroge WFM, Forkpa M, Bath E. Impact of racial discrimination on the mental health of minoritized youth. Curr Psychiatry Rep. 2021;23:81. https://doi.org/10.1007/s11920-021-01297-x.
Porche MV, Fortuna LR, Tolou-Shams M. Unpacking the layers: dismantling inequities in substance use services and outcomes for racially minoritized adolescents. Child Adolesc Psychiatr Clin N Am. 2022;31:223–36. https://doi.org/10.1016/j.chc.2021.11.002.
NIH’s Definition of a Clinical Trial. 2021. https://grants.nih.gov/policy/clinical-trials/definition.htm.
Kraft SA, Porter KM, Sullivan TR, Anderson EE, Garrison NA, Baker L, et al. Relationship building in pediatric research recruitment: insights from qualitative interviews with research staff. J Clin Transl Sci. 2022;6:e138. https://doi.org/10.1017/cts.2022.469.
Dedoose. 2018. www.dedoose.com.
Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15:398–405. https://doi.org/10.1111/nhs.12048.
Mazzocco MMM, Myers GF, Harum KH, Reiss AL. Children’s participation in genetic prevalence research: influences on enrollment and reports of parent satisfaction. J Appl Soc Psych. 1999;29:2308–27.
Langley JM, Halperin SA, Mills EL, Eastwood B. Parental willingness to enter a child in a controlled vaccine trial. Clin Invest Med. 1998;21:12–6.
Greenberg RG, Gamel B, Bloom D, Bradley J, Jafri HS, Hinton D, et al. Parents’ perceived obstacles to pediatric clinical trial participation: findings from the clinical trials transformation initiative. Contemp Clin Trials Commun. 2018;9:33–9.
Paquette E, Shukla A, Davidson J, Rychlik K, Davis M. Burden or opportunity? Parent experiences when approached for research in a pediatric intensive care unit. Ethics Hum Res. 2019;41:2–12. https://doi.org/10.1002/eahr.500014.
Morain SR, Joffe S, Largent EA. When is it ethical for physician-investigators to seek consent from their own patients? Am J Bioeth. 2019;19:11–18. https://doi.org/10.1080/15265161.2019.1572811.
Wulff A, Marschollek M. Learning healthcare systems in pediatrics: cross-institutional and data-driven decision-support for intensive care environments (CADDIE). Stud Health Technol Inf. 2018;251:109–12.
Tait AR, Voepel-Lewis T, Malviya S. Participation of children in clinical research: factors that influence a parent’s decision to consent. Anesthesiology. 2003;99:819–25. https://doi.org/10.1097/00000542-200310000-00012.
Hoberman A, Shaikh N, Bhatnagar S, Haralam MA, Kearney DH, Colborn DK, et al. Factors that influence parental decisions to participate in clinical research: consenters vs nonconsenters. JAMA Pediatr. 2013;167:561–6. https://doi.org/10.1001/jamapediatrics.2013.1050.
Jay F, Chantler T, Lees A, Pollard AJ. Children’s participation in vaccine research: parents’ views. Paediatr Nurs. 2007;19:14–8. https://doi.org/10.7748/paed2007.10.19.8.14.c4460.
Thomas M, Menon K. Consenting to pediatric critical care research: understanding the perspective of parents. Dynamics. 2013;24:18–24.
Weiss EM, Joffe S. Promoting informed decision making for comparative effectiveness randomized trials. JAMA Pediatr. 2015;169:803–4. https://doi.org/10.1001/jamapediatrics.2015.0906.
Tait AR, Voepel-Lewis T, Siewert M, Malviya S. Factors that influence parents’ decisions to consent to their child’s participation in clinical anesthesia research. Anesth Analg. 1998;86:50–3. https://doi.org/10.1097/00000539-199801000-00010.
Menon K, Ward RE, Gaboury I, Thomas M, Joffe A, Burns K, et al. Factors affecting consent in pediatric critical care research. Intensive Care Med. 2012;38:153–9. https://doi.org/10.1007/s00134-011-2412-0.
Korotchikova I, Boylan GB, Dempsey EM, Ryan CA. Presence of both parents during consent process in non‐therapeutic neonatal research increases positive response. Acta Paediatr. 2010;99:1484–8.
Mason SA, Allmark PJ. Obtaining informed consent to neonatal randomised controlled trials: interviews with parents and clinicians in the Euricon study. Lancet. 2000;356:2045–51. https://doi.org/10.1016/s0140-6736(00)03401-2.
Dickert NW, Bernard AM, Brabson JM, Hunter RJ, McLemore R, Mitchell AR, et al. Partnering with patients to bridge gaps in consent for acute care research. Am J Bioeth. 2020;20:7–17. https://doi.org/10.1080/15265161.2020.1745931.
Guttmann KF, Wu YW, Juul SE, Weiss EM. Consent related challenges for neonatal clinical trials. Am J Bioeth. 2020;20:38–40. https://doi.org/10.1080/15265161.2020.1745940.
Fisher HR, McKevitt C, Boaz A. Why do parents enrol their children in research: a narrative synthesis. J Med Ethics. 2011;37:544–51.
Cartwright K, Mahoney L, Ayers S, Rabe H. Parents’ perceptions of their infants’ participation in randomized controlled trials. J Obstet Gynecol Neonatal Nurs. 2011;40:555–65.
Chantler TE, Lees A, Moxon ER, Mant D, Pollard AJ, Fiztpatrick R. The role familiarity with science and medicine plays in parents’ decision making about enrolling a child in vaccine research. Qual Health Res. 2007;17:311–22. https://doi.org/10.1177/1049732306298561.
Bartlett A, Kolb SJ, Kingsley A, Swoboda KJ, Reyna SP, Sakonju A, et al. Recruitment & retention program for the NeuroNEXT SMA Biomarker Study: super babies for SMA! Contemp Clin Trials Commun. 2018;11:113–9. https://doi.org/10.1016/j.conctc.2018.07.002.
Harth S, Thong Y. Sociodemographic and motivational characteristics of parents who volunteer their children for clinical research: a controlled study. BMJ. 1990;300:1372–5.
Chappuy H, Doz F, Blanche S, Gentet JC, Pons G, Tréluyer JM. Parental consent in paediatric clinical research. Arch Dis Child. 2006;91:112–6. https://doi.org/10.1136/adc.2005.076141.
Koenig BA. Have we asked too much of consent?. Hastings Cent Rep. 2014;44:33–4. https://doi.org/10.1002/hast.329.
Grady C. Enduring and emerging challenges of informed consent. N Engl J Med. 2015;372:855–62. https://doi.org/10.1056/NEJMra1411250.
Faden RR, Kass NE, Goodman SN, Pronovost P, Tunis S, Beauchamp TL. An ethics framework for a learning health care system: a departure from traditional research ethics and clinical ethics. Hastings Cent Rep. 2013:S16–27. https://doi.org/10.1002/hast.134.
Faden RR, Beauchamp TL, Kass NE. Informed consent for comparative effectiveness trials. N Engl J Med. 2014;370:1959–60. https://doi.org/10.1056/NEJMc1403310.
Kim SY, Miller FG. Informed consent for pragmatic trials-the integrated consent model. N Engl J Med. 2014;370:769–72. https://doi.org/10.1056/NEJMhle1312508.
Acknowledgements
We thank the parents who participated in our interviews. We thank our neonatal research collaborators who referred parents to us, including: Matthew W. Harer, MD, Department of Pediatrics, Division of Neonatology, University of Wisconsin School of Medicine and Public Health; Sarah E Kolnik MD MBA, Department of Pediatrics, University of Washington; Dennis E. Mayock, MD, Department of Pediatrics, University of Washington; Mar Romero Lopez, MD, Department of Pediatrics, McGovern Medical School, the University of Texas Health Science Center; Jon E. Tyson, MD, MPH, Professor, Vice Dean for Clinical Research and Healthcare Quality; Michelle Bain Distinguished Professor in Medicine and Public Health; Susan H. Wootton, MD, Pediatric Infectious Diseases, McGovern Medical School at UTHealth. We thank members of Dr. Weiss’s NICU Parent advisory panel for their important contributions in the design of our interview guide and interpretation of our interview findings. We thank members of Dr. Weiss’s K23 expert advisory panel for their conceptual and logistical support. We thank research team members for coding, including: Hannah S. Lewis, BA, Elson S. Floyd College of Medicine, Washington State University. None of the above non-author contributors were provided monetary compensation. We have obtained written permission to include their names in the Acknowledgement section of this manuscript. EMW had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All results presented in the manuscript are original and have not been published previously.
Funding
This study was supported by National Institutes of Health through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23HD103872, supporting Elliott Weiss). Additional support was provided by the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR00231, supporting BSW and KMP).
Author information
Authors and Affiliations
Contributions
EMW conceptualized the study, designed the study, carried out the analysis, drafted the initial manuscript, and reviewed and critically revised the manuscript for important intellectual content. KMP designed the data collection instruments, carried out the analysis, and reviewed and critically revised the manuscript for important intellectual content. EO carried out the analysis and reviewed and critically revised the manuscript for important intellectual content. MP-D, PKD, SLM, and ES helped design the data collection instruments, identified potential participants, supported the analysis and interpretation of data, and reviewed and critically revised the manuscript for important intellectual content. AM designed the data collection instruments, recruited for and performed the interviews, carried out the analysis, and reviewed and critically revised the manuscript for important intellectual content. BSW conceptualized the study, designed the study, carried out the analysis, and reviewed and critically revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This was approved by the Seattle Children’s Hospital Institutional Review Board and was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weiss, E.M., Porter, K.M., Oslin, E. et al. Experiences and preferences for learning about neonatal research: insights from parent interviews. J Perinatol 44, 404–414 (2024). https://doi.org/10.1038/s41372-023-01790-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01790-6