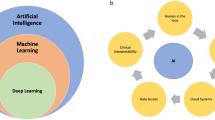
Abstract
Artificial intelligence (AI) offers tremendous potential to transform neonatology through improved diagnostics, personalized treatments, and earlier prevention of complications. However, there are many challenges to address before AI is ready for clinical practice. This review defines key AI concepts and discusses ethical considerations and implicit biases associated with AI. Next we will review literature examples of AI already being explored in neonatology research and we will suggest future potentials for AI work. Examples discussed in this article include predicting outcomes such as sepsis, optimizing oxygen therapy, and image analysis to detect brain injury and retinopathy of prematurity. Realizing AI’s potential necessitates collaboration between diverse stakeholders across the entire process of incorporating AI tools in the NICU to address testability, usability, bias, and transparency. With multi-center and multi-disciplinary collaboration, AI holds tremendous potential to transform the future of neonatology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rowe M. An introduction to machine learning for clinicians. Acad Med. 2019;94:1433–6.
Beam AL, Kohane IS. Big data and machine learning in health care. JAMA 2018;319:1317–8.
Hoodbhoy Z, Masroor Jeelani S, Aziz A, Habib MI, Iqbal B, Akmal W, et al. Machine learning for child and adolescent health: A systematic review. Pediatrics. (2021) Jan;147.
Kwok TC, Henry C, Saffaran S, Meeus M, Bates D, Van Laere D, et al. Application and potential of artificial intelligence in neonatal medicine. Semin Fetal Neonatal Med. (2022) Apr 18;101346.
Haug CJ, Drazen JM. Artificial intelligence and machine learning in clinical medicine, 2023. N Engl J Med. 2023;388:1201–8.
Sun Q, Zou X, Yan Y, Zhang H, Wang S, Gao Y, et al. Machine learning-based prediction model of preterm birth using electronic health record. J Health Eng. 2022;2022:9635526.
Abraham A, Le B, Kosti I, Straub P, Velez-Edwards DR, Davis LK, et al. Dense phenotyping from electronic health records enables machine learning-based prediction of preterm birth. BMC Med. 2022;20:333.
Weber A, Darmstadt GL, Gruber S, Foeller ME, Carmichael SL, Stevenson DK, et al. Application of machine-learning to predict early spontaneous preterm birth among nulliparous non-Hispanic black and white women. Ann Epidemiol. 2018;28:783–9.e1.
Gao C, Osmundson S, Velez Edwards DR, Jackson GP, Malin BA, Chen Y. Deep learning predicts extreme preterm birth from electronic health records. J Biomed Inf. 2019;100:103334.
Jehan F, Sazawal S, Baqui AH, Nisar MI, Dhingra U, Khanam R, et al. Multiomics characterization of preterm birth in low- and middle-income countries. JAMA Netw Open. 2020;3:e2029655.
Wynn JL, Polin RA. Progress in the management of neonatal sepsis: the importance of a consensus definition. Pediatr Res. 2018;83:13–5.
Moorman JR, Carlo WA, Kattwinkel J, Schelonka RL, Porcelli PJ, Navarrete CT, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatr. 2011;159:900–6.e1.
Fairchild KD, Schelonka RL, Kaufman DA, Carlo WA, Kattwinkel J, Porcelli PJ, et al. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatr Res. 2013;74:570–5.
Peng Z, Varisco G, Liang R-H, Kommers D, Cottaar W, Andriessen P, et al. DeepLOS: deep learning for late-onset sepsis prediction in preterm infants using heart rate variability. Smart Health. 2022;26:100335.
Kausch SL, Brandberg JG, Qiu J, Panda A, Binai A, Isler J, et al. Cardiorespiratory signature of neonatal sepsis: development and validation of prediction models in 3 NICUs. Pediatr Res. 2023;93:1913–21.
Cabrera-Quiros L, Kommers D, Wolvers MK, Oosterwijk L, Arents N, van der Sluijs-Bens J, et al. Prediction of late-onset sepsis in preterm infants using monitoring signals and machine learning. Crit Care Explor. 2021;3:e0302.
Puopolo KM, Draper D, Wi S, Newman TB, Zupancic J, Lieberman E, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics. 2011;128:e1155–63.
Escobar GJ, Puopolo KM, Wi S, Turk BJ, Kuzniewicz MW, Walsh EM, et al. Stratification of risk of early-onset sepsis in newborns ≥ 34 weeks’ gestation. Pediatrics. 2014;133:30–6.
Kuzniewicz MW, Puopolo KM, Fischer A, Walsh EM, Li S, Newman TB, et al. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr. 2017;171:365–71.
Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2018;103:F182–9.
Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
Battersby C, Longford N, Costeloe K, Modi N, UK Neonatal Collaborative Necrotising Enterocolitis Study Group. Development of a gestational age-specific case definition for neonatal necrotizing enterocolitis. JAMA Pediatr. 2017;171:256–63.
Lure AC, Du X, Black EW, Irons R, Lemas DJ, Taylor JA, et al. Using machine learning analysis to assist in differentiating between necrotizing enterocolitis and spontaneous intestinal perforation: a novel predictive analytic tool. J Pediatr Surg. 2021;56:1703–10.
Son J, Kim D, Na JY, Jung D, Ahn J-H, Kim TH, et al. Development of artificial neural networks for early prediction of intestinal perforation in preterm infants. Sci Rep. 2022;12:12112.
Meister AL, Gardner FC, Browning KN, Travagli RA, Palmer C, Doheny KK. Vagal tone and proinflammatory cytokines predict feeding intolerance and necrotizing enterocolitis risk. Adv Neonatal Care. 2021;21:452–61.
Doheny KK, Palmer C, Browning KN, Jairath P, Liao D, He F, et al. Diminished vagal tone is a predictive biomarker of necrotizing enterocolitis-risk in preterm infants. Neurogastroenterol Motil. 2014;26:832–40.
Stone ML, Tatum PM, Weitkamp JH, Mukherjee AB, Attridge J, McGahren ED, et al. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. J Perinatol. 2013;33:847–50.
Lin YC, Salleb-Aouissi A, Hooven TA. Interpretable prediction of necrotizing enterocolitis from machine learning analysis of premature infant stool microbiota. BMC Bioinforma. 2022;23:104.
Rusconi B, Jiang X, Sidhu R, Ory DS, Warner BB, Tarr PI. Gut sphingolipid composition as a prelude to necrotizing enterocolitis. Sci Rep. 2018;8:10984.
Sylvester KG, Ling XB, Liu GY, Kastenberg ZJ, Ji J, Hu Z, et al. A novel urine peptide biomarker-based algorithm for the prognosis of necrotising enterocolitis in human infants. Gut. 2014;63:1284–92.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–51.
Williams E, Greenough A. Advances in treating bronchopulmonary dysplasia. Expert Rev Respir Med. 2019;13:727–35.
Principi N, Di Pietro GM, Esposito S. Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. J Transl Med. 2018;16:36.
He W, Zhang L, Feng R, Fang W-H, Cao Y, Sun S-Q, et al. Risk factors and machine learning prediction models for bronchopulmonary dysplasia severity in the Chinese population. World J Pediatr. 2023;19:568–76.
Valenzuela-Stutman D, Marshall G, Tapia JL, Mariani G, Bancalari A, Gonzalez Á, et al. Bronchopulmonary dysplasia: risk prediction models for very-low- birth-weight infants. J Perinatol. 2019;39:1275–81.
Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–22.
Dai D, Chen H, Dong X, Chen J, Mei M, Lu Y, et al. Bronchopulmonary dysplasia predicted by developing a machine learning model of genetic and clinical information. Front Genet. 2021;12:689071.
Leigh RM, Pham A, Rao SS, Vora FM, Hou G, Kent C, et al. Machine learning for prediction of bronchopulmonary dysplasia-free survival among very preterm infants. BMC Pediatr. 2022;22:542.
Jia M, Li J, Zhang J, Wei N, Yin Y, Chen H, et al. Identification and validation of cuproptosis related genes and signature markers in bronchopulmonary dysplasia disease using bioinformatics analysis and machine learning. BMC Med Inf Decis Mak. 2023;23:69.
Fairchild KD, Nagraj VP, Sullivan BA, Moorman JR, Lake DE. Oxygen desaturations in the early neonatal period predict development of bronchopulmonary dysplasia. Pediatr Res. 2019;85:987–93.
Moreira A, Tovar M, Smith AM, Lee GC, Meunier JA, Cheema Z, et al. Development of a peripheral blood transcriptomic gene signature to predict bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2023;324:L76–87.
Sullivan BA, McClure C, Hicks J, Lake DE, Moorman JR, Fairchild KD. Early heart rate characteristics predict death and morbidities in preterm infants. J Pediatr. 2016;174:57–62.
Sullivan BA, Wallman-Stokes A, Isler J, Sahni R, Moorman JR, Fairchild KD, et al. Early pulse oximetry data improves prediction of death and adverse outcomes in a two-center cohort of very low birth weight infants. Am J Perinatol. 2018;35:1331–8.
Hartnett ME. Pathophysiology of retinopathy of prematurity. Annu Rev Vis Sci. 2023;9:39–70.
Di Fiore JM, Kaffashi F, Loparo K, Sattar A, Schluchter M, Foglyano R, et al. The relationship between patterns of intermittent hypoxia and retinopathy of prematurity in preterm infants. Pediatr Res. 2012;72:606–12.
Di Fiore JM, Bloom JN, Orge F, Schutt A, Schluchter M, Cheruvu VK, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 2010;157:69–73.
Athikarisamy S, Desai S, Patole S, Rao S, Simmer K, Lam GC. The use of postnatal weight gain algorithms to predict severe or type 1 retinopathy of prematurity: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:e2135879.
Wang J, Ji J, Zhang M, Lin J-W, Zhang G, Gong W, et al. Automated explainable multidimensional deep learning platform of retinal images for retinopathy of prematurity screening. JAMA Netw Open. 2021;4:e218758.
Brown JM, Campbell JP, Beers A, Chang K, Ostmo S, Chan RVP, et al. Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol. 2018;136:803–10.
Zekavat SM, Raghu VK, Trinder M, Ye Y, Koyama S, Honigberg MC, et al. Deep learning of the retina enables phenome- and genome-wide analyses of the microvasculature. Circulation. 2022;145:134–50.
Ho T, Dukhovny D, Zupancic JAF, Goldmann DA, Horbar JD, Pursley DM. Choosing wisely in newborn medicine: five opportunities to increase value. Pediatrics. 2015;136:e482–9.
Mohammad K, Scott JN, Leijser LM, Zein H, Afifi J, Piedboeuf B, et al. Consensus approach for standardizing the screening and classification of preterm brain injury diagnosed with cranial ultrasound: a canadian perspective. Front Pediatr. 2021;9:618236.
Kidokoro H, Neil JJ, Inder TE, New MR. imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013;34:2208–14.
Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–9.
Trivedi SB, Vesoulis ZA, Rao R, Liao SM, Shimony JS, McKinstry RC, et al. A validated clinical MRI injury scoring system in neonatal hypoxic-ischemic encephalopathy. Pediatr Radio. 2017;47:1491–9.
Weeke LC, Groenendaal F, Mudigonda K, Blennow M, Lequin MH, Meiners LC, et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J Pediatr. 2018;192:33–40.e2.
Gruber N, Galijasevic M, Regodic M, Grams AE, Siedentopf C, Steiger R, et al. A deep learning pipeline for the automated segmentation of posterior limb of internal capsule in preterm neonates. Artif Intell Med. 2022;132:102384.
Richter L, Fetit AE. Accurate segmentation of neonatal brain MRI with deep learning. Front Neuroinformatics 2022;16:1006532.
Shen DD, Bao SL, Wang Y, Chen YC, Zhang YC, Li XC, et al. An automatic and accurate deep learning-based neuroimaging pipeline for the neonatal brain. Pediatr Radio. 2023;53:1685–97.
Weiss RJ, Bates SV, Song Y, Zhang Y, Herzberg EM, Chen Y-C, et al. Mining multi-site clinical data to develop machine learning MRI biomarkers: application to neonatal hypoxic ischemic encephalopathy. J Transl Med. 2019;17:385.
Mathieson SR, Stevenson NJ, Low E, Marnane WP, Rennie JM, Temko A, et al. Validation of an automated seizure detection algorithm for term neonates. Clin Neurophysiol. 2016;127:156–68.
Pavel AM, Rennie JM, de Vries LS, Blennow M, Foran A, Shah DK, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4:740–9.
Raurale SA, Boylan GB, Mathieson SR, Marnane WP, Lightbody G, O’Toole JM. Grading hypoxic-ischemic encephalopathy in neonatal EEG with convolutional neural networks and quadratic time-frequency distributions. J Neural Eng. 2021;18:046007.
Pavel AM, O’Toole JM, Proietti J, Livingstone V, Mitra S, Marnane WP, et al. Machine learning for the early prediction of infants with electrographic seizures in neonatal hypoxic-ischemic encephalopathy. Epilepsia 2023;64:456–68.
Pliego J. Surgical correction of mitral valve stenosis under direct vision using extracorporeal circulation. Gac Med Mex. 1964;94:423–33.
O’Shea A, Lightbody G, Boylan G, Temko A. Neonatal seizure detection from raw multi-channel EEG using a fully convolutional architecture. Neural Netw. 2020;123:12–25.
Raeisi K, Khazaei M, Croce P, Tamburro G, Comani S, Zappasodi F. A graph convolutional neural network for the automated detection of seizures in the neonatal EEG. Comput Methods Prog Biomed. 2022;222:106950.
Srinivasakumar P, Zempel J, Trivedi S, Wallendorf M, Rao R, Smith B, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics 2015;136:e1302–9.
Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J Pediatr. 2009;155:318–23.
Hartnett ME, Lane RH. Effects of oxygen on the development and severity of retinopathy of prematurity. J AAPOS. 2013;17:229–34.
Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, et al. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA. 2018;319:2190–201.
Ruppel H, Makeneni S, Faerber JA, Lane-Fall MB, Foglia EE, O’Byrne ML, et al. Evaluating the accuracy of pulse oximetry in children according to race. JAMA Pediatr. 2023;177:540–3.
Vesoulis Z, Tims A, Lodhi H, Lalos N, Whitehead H. Racial discrepancy in pulse oximeter accuracy in preterm infants. J Perinatol. 2022;42:79–85.
Alvarez D, Hornero R, Abásolo D, del Campo F, Zamarrón C. Nonlinear characteristics of blood oxygen saturation from nocturnal oximetry for obstructive sleep apnoea detection. Physiol Meas. 2006;27:399–412.
Lu X, Jiang L, Chen T, Wang Y, Zhang B, Hong Y, et al. Continuously available ratio of SpO2/FiO2 serves as a noninvasive prognostic marker for intensive care patients with COVID-19. Respir Res. 2020;21:194.
Sadeghi Fathabadi O, Gale TJ, Lim K, Salmon BP, Dawson JA, Wheeler KI, et al. Characterisation of the oxygenation response to inspired oxygen adjustments in preterm infants. Neonatology 2016;109:37–43.
Ostojic D, Guglielmini S, Moser V, Fauchère JC, Bucher HU, Bassler D, et al. Reducing false alarm rates in neonatal intensive care: a new machine learning approach. Adv Exp Med Biol. 2020;1232:285–90.
Kristiansen TB, Kristensen K, Uffelmann J, Brandslund I. Erroneous data: the Achilles’ heel of AI and personalized medicine. Front Digit Health. 2022;4:862095.
Elmore JG, Lee CI. Data quality, data sharing, and moving artificial intelligence forward. JAMA Netw Open. 2021;4:e2119345.
Miller DD. The medical AI insurgency: what physicians must know about data to practice with intelligent machines. npj Digital Med. 2019;2:62.
Scott IA, Carter SM, Coiera E. Exploring stakeholder attitudes towards AI in clinical practice. BMJ Health Care Inform. 2021;28:e100450.
Ghassemi M, Oakden-Rayner L, Beam AL. The false hope of current approaches to explainable artificial intelligence in health care. Lancet Digit Health. 2021;3:e745–50.
Gerke S, Babic B, Evgeniou T, Cohen IG. The need for a system view to regulate artificial intelligence/machine learning-based software as medical device. npj Digital Med. 2020;3:53.
TerKonda SP, Fish EM. Artificial intelligence viewed through the lens of state regulation. Intell Based Med. 2023;7:100088.
Kappen TH, van Klei WA, van Wolfswinkel L, Kalkman CJ, Vergouwe Y, Moons KGM. Evaluating the impact of prediction models: lessons learned, challenges, and recommendations. Diagn Progn Res. 2018;2:11.
Pencina MJ, Goldstein BA, D’Agostino RB. Prediction models - development, evaluation, and clinical application. N Engl J Med. 2020;382:1583–6.
Parikh RB, Helmchen LA. Paying for artificial intelligence in medicine. npj Digital Med. 2022;5:63.
Chen MM, Golding LP, Nicola GN. Who will pay for AI? Radiol. Artif Intell. 2021;3:e210030.
Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial bias in pulse oximetry measurement. N. Engl J Med. 2020;383:2477–8.
Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev. 2000;21:75–90.
Martin AE, D’Agostino JA, Passarella M, Lorch SA. Racial differences in parental satisfaction with neonatal intensive care unit nursing care. J Perinatol. 2016;36:1001–7.
Sullivan BA, Doshi A, Chernyavskiy P, Husain A, Binai A, Sahni R, et al. Neighborhood deprivation and association with neonatal intensive care unit mortality and morbidity for extremely premature infants. JAMA Netw Open. 2023;6:e2311761.
Travers CP, Carlo WA, McDonald SA, Das A, Ambalavanan N, Bell EF, et al. Racial/ethnic disparities among extremely preterm infants in the united states from 2002 to 2016. JAMA Netw Open. 2020;3:e206757.
Esteban-Escaño J, Castán B, Castán S, Chóliz-Ezquerro M, Asensio C, Laliena AR, et al. Machine learning algorithm to predict acidemia using electronic fetal monitoring recording parameters. Entropy. 2021;24:68.
Goldstein B, Fiser DH, Kelly MM, Mickelsen D, Ruttimann U, Pollack MM. Decomplexification in critical illness and injury: relationship between heart rate variability, severity of illness, and outcome. CritCare Med. 1998;26:352–7.
Ellenby MS, McNames J, Lai S, McDonald BA, Krieger D, Sclabassi RJ, et al. Uncoupling and recoupling of autonomic regulation of the heart beat in pediatric septic shock. Shock 2001;16:274–7.
Badke CM, Marsillio LE, Weese-Mayer DE, Sanchez-Pinto LN. Autonomic nervous system dysfunction in pediatric sepsis. Front Pediatr. 2018;6:280.
Papaioannou VE, Maglaveras N, Houvarda I, Antoniadou E, Vretzakis G. Investigation of altered heart rate variability, nonlinear properties of heart rate signals, and organ dysfunction longitudinally over time in intensive care unit patients. J Crit Care. 2006;21:95–103.
Griffin MP, Lake DE, Bissonette EA, Harrell FE, O’Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics 2005;116:1070–4.
Goddard K, Roudsari A, Wyatt JC. Automation bias: a systematic review of frequency, effect mediators, and mitigators. J Am Med Inf Assoc. 2012;19:121–7.
Kligfield P, Gettes LS, Bailey JJ, Childers R, Deal BJ, Hancock EW, et al. Recommendations for the standardization and interpretation of the electrocardiogram: part I: The electrocardiogram and its technology: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2007;115:1306–24.
Siontis KC, Noseworthy PA, Attia ZI, Friedman PA. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat Rev Cardiol. 2021;18:465–78.
Wysocki O, Davies JK, Vigo M, Armstrong AC, Landers D, Lee R, et al. Assessing the communication gap between AI models and healthcare professionals: Explainability, utility and trust in AI-driven clinical decision-making. Artif Intell. 2023;316:103839.
Global digital health market forecast 2025 | Statista [Internet]. 2023. Available from: https://www.statista.com/statistics/1092869/global-digital-health-market-size-forecast/
Torous J, Stern AD, Bourgeois FT. Regulatory considerations to keep pace with innovation in digital health products. npj Digital Med. 2022;5:121.
Artificial Intelligence and Machine Learning in Software as a Medical Device | FDA [Internet]. 2023. Available from: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device
Author information
Authors and Affiliations
Contributions
RMM contributed to the conception and design. All authors contributed to the first draft. RMM, KB, ZAV, and BAS revised the article. ANH and KBA created the Figures. All authors contributed a final revision of the article, including providing intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sullivan, B.A., Beam, K., Vesoulis, Z.A. et al. Transforming neonatal care with artificial intelligence: challenges, ethical consideration, and opportunities. J Perinatol 44, 1–11 (2024). https://doi.org/10.1038/s41372-023-01848-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01848-5