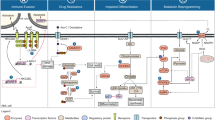
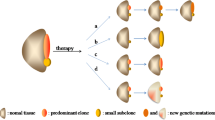
Abstract
Precision medicine is gaining importance in the treatment of acute myeloid leukemia (AML). Objectively reviewing past and current knowledge aids guiding future research. Therefore, we provide a complete overview of all phase II and phase III trials investigating targeted therapies in AML and their primary endpoints over the past two decades in perspective of their clinical benefit. We assessed whether drugs were primarily designed to treat AML or were repurposed and how successful they were based on progression of distinct drugs from phase II to phase III to FDA-approval. Between January 2000 and September 2020, 167 agents with 96 targets were investigated in 397 phase II trials. Twenty-eight agents were steered towards phase III, after three phase II trials on average. Repurposed drugs less often advanced in clinical development than drugs primarily developed for AML. Composite responses were the most prevalent primary endpoints in phase II. Of the eight FDA-approved drugs, none investigated quality of life at time of approval, and three out of eight have yet to show benefit in overall survival. Returns on targeted therapy research remain lean for AML patients. Future trials should not overlook non-targeted agents and foremost study endpoints proven to predict patient well-being.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mauro MJ, O’Dwyer ME, Druker BJ. ST1571, a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia: validating the promise of molecularly targeted therapy. Cancer Chemother Pharmacol. 2001;48 Suppl 1:S77–8.
Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J Hematol Oncol. 2019;12:100.
Hilal T. Progress in acute myeloid leukaemia: small molecular inhibitors with small benefits. Ecancermedicalscience. 2020;14:1015.
Prasad V, Fojo T, Brada M. Precision oncology: origins, optimism, and potential. Lancet Oncol. 2016;17:e81–6.
Wouters OJ, McKee M, Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. JAMA. 2020;323:844–53.
Cherny NI, Dafni U, Bogaerts J, Latino NJ, Pentheroudakis G, Douillard JY, et al. ESMO-magnitude of clinical benefit scale version 1.1. Ann Oncol. 2017;28:2340–66.
Kiesewetter B, Cherny NI, Boissel N, Cerisoli F, Dafni U, de Vries EGE, et al. EHA evaluation of the ESMO— Magnitude of Clinical Benefit Scale version 1.1 (ESMO-MCBS v1.1) for haematological malignancies. ESMO Open 2020;5:e000611.
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–29.
Tran AA, Miljkovic M, Prasad V. Analysis of estimated clinical benefit of newly approved drugs for US patients with acute myeloid leukemia. Leuk Res. 2020;96:106420.
Aziz H, Ping CY, Alias H, Ab Mutalib NS, Jamal R. Gene mutations as emerging biomarkers and therapeutic targets for relapsed acute myeloid leukemia. Front Pharmacol. 2017;8:897.
Zeijlemaker W, Gratama JW, Schuurhuis GJ. Tumor heterogeneity makes AML a “moving target” for detection of residual disease. Cytom B Clin Cytom. 2014;86:3–14.
Daver N, Cortes J, Ravandi F, Patel KP, Burger JA, Konopleva M, et al. Secondary mutations as mediators of resistance to targeted therapy in leukemia. Blood. 2015;125:3236–45.
Abadir E, Gasiorowski RE, Silveira PA, Larsen S, Clark GJ. Is hematopoietic stem cell transplantation required to unleash the full potential of immunotherapy in acute myeloid leukemia? J Clin Med. 2020;9:554.
Seruga B, Ocana A, Amir E, Tannock IF. Failures in phase III: causes and consequences. Clin Cancer Res. 2015;21:4552–60.
Medeiros BC. Interpretation of clinical endpoints in trials of acute myeloid leukemia. Leuk Res. 2018;68:32–9.
Prasad V, Booth CM. Multiplicity in oncology randomised controlled trials: a threat to medical evidence? Lancet Oncol. 2019;20:1638–40.
Walter RB, Appelbaum FR, Tallman MS, Weiss NS, Larson RA, Estey EH. Shortcomings in the clinical evaluation of new drugs: acute myeloid leukemia as paradigm. Blood. 2010;116:2420–8.
Sukhai MA, Spagnuolo PA, Weir S, Kasper J, Patton L, Schimmer AD. New sources of drugs for hematologic malignancies. Blood. 2011;117:6747–55.
Othus M, van Putten W, Lowenberg B, Petersdorf SH, Nand S, Erba H, et al. Relationship between event-free survival and overall survival in acute myeloid leukemia: a report from SWOG, HOVON/SAKK, and MRC/NCRI. Haematologica. 2016;101:e284–6.
Kemp R, Prasad V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 2017;15:134.
Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175:1389–98.
Walter RB, Kantarjian HM, Huang X, Pierce SA, Sun Z, Gundacker HM, et al. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: a combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study. J Clin Oncol. 2010;28:1766–71.
Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med. 2019;381:1728–40.
Zeidan AM, Pandya BJ, Qi CZ, Garnham A, Yang H, Shah MV. Cost-effectiveness analysis of gilteritinib versus best supportive care (BSC) for the treatment of relapsed or refractory (R/R) FLT3 mutation-positive (FLT3mut+) acute myeloid leukemia (AML). Blood. 2019;134 (Supplement_1):5085.
Stein E, Xie J, Duchesneau E, Bhattacharyya S, Vudumula U, Ndife B, et al. Cost effectiveness of midostaurin in the treatment of newly diagnosed FLT3-mutated acute myeloid leukemia in the United States. Pharmacoeconomics. 2019;37:239–53.
Sertkaya A, Wong HH, Jessup A, Beleche T. Key cost drivers of pharmaceutical clinical trials in the United States. Clin Trials. 2016;13:117–26.
Berry S, Carlin B, Lee J, Muller P. Bayesian adaptive methods for clinical trials. Boca Raton: CRC Press; 2011.
Tran A, Klossner Q, Crain T, Prasad V. Shifting, overlapping and expanding use of “precision oncology” terminology: a retrospective literature analysis. BMJ Open. 2020;10:e036357.
Prasad V, Gale RP. Precision medicine in acute myeloid leukemia: hope, hype or both? Leuk Res. 2016;48:73–7.
Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie JN, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–16.
Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33:379–89.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377:454–64.
Pemmaraju N, Lane AA, Sweet KL, Stein AS, Vasu S, Blum W, et al. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2019;380:1628–37.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17.
Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–31.
DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378:2386–98.
Acknowledgements
The authors thank Noor Gieles for extensive proof reading.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cucchi, D.G.J., Polak, T.B., Ossenkoppele, G.J. et al. Two decades of targeted therapies in acute myeloid leukemia. Leukemia 35, 651–660 (2021). https://doi.org/10.1038/s41375-021-01164-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01164-x
This article is cited by
-
Nuclear factor I-C overexpression promotes monocytic development and cell survival in acute myeloid leukemia
Leukemia (2023)
-
Clonal evolution in leukemia: preleukemia, evolutionary models, and clinical implications
Genome Instability & Disease (2023)
-
Imetelstat-mediated alterations in fatty acid metabolism to induce ferroptosis as a therapeutic strategy for acute myeloid leukemia
Nature Cancer (2023)
-
Graphdiyne oxide nanosheets display selective anti-leukemia efficacy against DNMT3A-mutant AML cells
Nature Communications (2022)
-
Context-specific effects of NOX4 inactivation in acute myeloid leukemia (AML)
Journal of Cancer Research and Clinical Oncology (2022)