Abstract
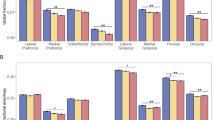
Abnormalities in brain white matter (WM) are reported in youth at-risk for psychosis. Yet, the neurodevelopmental time course of these abnormalities remains unclear. Thus, longitudinal diffusion-weighted imaging (DWI) was used to investigate WM abnormalities in youth at-risk for psychosis. A subset of individuals from the Philadelphia Neurodevelopmental Cohort (PNC) completed two DWI scans approximately 20 months apart. Youths were identified through structured interview as having subthreshold persistent psychosis risk symptoms (n = 46), and were compared to healthy typically developing participants (TD; n = 98). Analyses were conducted at voxelwise and regional levels. Nonlinear developmental patterns were examined using penalized splines within a generalized additive model. Compared to TD, youth with persistent psychosis risk symptoms had lower whole-brain WM fractional anisotropy (FA) and higher radial diffusivity (RD). Voxelwise analyses revealed clusters of significant WM abnormalities within the temporal and parietal lobes. Lower FA within the cingulum bundle of hippocampus and cerebrospinal tracts were the most robust deficits in individuals with persistent psychosis symptoms. These findings were consistent over two visits. Thus, it appears that WM abnormalities are present early in youth with persistent psychosis risk symptoms, however, there is little evidence to suggest that these features emerge in late adolescence or early adulthood. Future studies should seek to characterize WM abnormalities in younger individuals and follow individuals as subthreshold psychotic symptoms emerge.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–38.
Zeidler J, Slawik L, Fleischmann J, Greiner W. The costs of schizophrenia and predictors of hospitalisation from the statutory health insurance perspective. Health Econ Rev. 2012;2:1–8.
Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, et al. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry. 2000;57:769–75.
Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25.
Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia. Ann N Y Acad Sci. 2006;1003:138–58.
Marsman A, van den Heuvel MP, Klomp DWJ, Kahn RS, Luijten PR, Pol HEH. Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophr Bull. 2011;39:120–9.
Kyriakopoulos M, Bargiotas T, Barker GJ, Frangou S. Diffusion tensor imaging in schizophrenia. Eur Psychiatry. 2008;23:255–73.
Roalf DR, Ruparel K, Verma R, Elliott MA, Gur RE, Gur RC. White matter organization and neurocognitive performance variability in schizophrenia. Schizophr Res. 2013;143:172–8.
McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–48.
Pettersson-Yeo W, Benetti S, Marquand A, Dell‚ÄòAcqua F, Williams S, Allen P, et al. Using genetic, cognitive and multi-modal neuroimaging data to identify ultra-high-risk and first-episode psychosis at the individual level. Psychol Med. 2013;43:1–16.
Barbato M, Colijn MA, Keefe RS, Perkins DO, Woods SW, Hawkins KA, et al. The course of cognitive functioning over six months in individuals at clinical high risk for psychosis. Psychiatry Res. 2012;206:195–9.
Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37.
Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, et al. The psychosis spectrum in a young US community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13:296–305.
Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, et al. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. 2015;56:1356–69.
Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220.
Roalf D, Nanga R, Rupert P, Hariharan H, Quarmley M, Calkins M, et al. Glutamate imaging (GluCEST) reveals lower brain GluCEST contrast in patients on the psychosis spectrum. Mol Psychiatry. 2017;22:1298–305.
Chan RC, Di X, McAlonan GM, Gong Q-y. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37:177–88.
Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 2009;66:562–9.
Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259.
Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–46.
Epstein KA, Cullen K, Mueller B, Lee S, Kumre S. White matter abnormalities and cognitive impairment in early-onset schizophrenia-spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2013;53:362–72.
White T, Kendi ATK, Lehéricy S, Kendi M, Karatekin C, Guimaraes A, et al. Disruption of hippocampal connectivity in children and adolescents with schizophrenia—a voxel-based diffusion tensor imaging study. Schizophr Res. 2007;90:302–7.
Kelly S, Agartz N, Andreassen OA, Fatourous-Bergman H, Brouwer R, Cahn W, et al. White matter differences in schizophrenia: meta-analytic findings from ENIGMA-schizophrenia DTI. Mol Psychiatry. 2018;23:1261–9.
Cooper S, Alm KH, Olson IR, Ellman LM. White matter alterations in individuals experiencing attenuated positive psychotic symptoms. Early Interv Psychiatry. 2018;12:372–9.
Krakauer K, Ebdrup B, Glenthøj B, Raghava J, Nordholm D, Randers L, et al. Patterns of white matter microstructure in individuals at ultra-high-risk for psychosis: associations to level of functioning and clinical symptoms. Psychol Med. 2017;47:2689–707.
Samartzis L, Dima D, Fusar‐Poli P, Kyriakopoulos M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J Neuroimaging. 2014;24:101–10.
Tamnes CK, Agartz I. White matter microstructure in early-onset schizophrenia: a systematic review of diffusion tensor imaging studies. J Am Acad Child Adolesc Psychiatry. 2016;55:269–79.
Douaud G, Mackay C, Andersson J, James S, Quested D, Ray MK, et al. Schizophrenia delays and alters maturation of the brain in adolescence. Brain. 2009;132:2437–48.
Epstein KA, Kumra S. White matter fractional anisotropy over two time points in early onset schizophrenia and adolescent cannabis use disorder: a naturalistic diffusion tensor imaging study. Psychiatry Res. 2015;232:34–41.
Saito J, Hori M, Nemoto T, Katagiri N, Shimoji K, Ito S, et al. Longitudinal study examining abnormal white matter integrity using a tract‐specific analysis in individuals with a high risk for psychosis. Psychiatry Clin Neurosci. 2017;71:530–41.
Krakauer K, Nordentoft M, Glenthøj B, Raghava J, Nordholm D, Randers L, et al. White matter maturation during 12 months in individuals at ultra‐high‐risk for psychosis. Acta Psychiatr Scand. 2018;137:65–78.
Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014;86:544–53.
Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, et al. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry. 2014;71:366–74.
Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, et al. The Philadelphia Neurodevelopmental Cohort: a publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 2016;124:1115–9.
Moore T, Martin I, Gur O, Jackson C, Scott J, Calkins M, et al. Characterizing social environment’s association with neurocognition using census and crime data linked to the Philadelphia Neurodevelopmental Cohort. Psychol Med. 2016;46:599–610.
Calkins ME, Moore TM, Satterthwaite TD, Wolf DH, Turetsky BI, Roalf DR, et al. Persistence of psychosis spectrum symptoms in the Philadelphia Neurodevelopmental Cohort: a prospective two‐year follow‐up. World Psychiatry. 2017;16:62–76.
Turetsky BI, Moberg PJ, Quarmley M, Dress E, Calkins ME, Ruparel K, et al. Structural anomalies of the peripheral olfactory system in psychosis high-risk subjects. Schizophr Res. 2018;195:197–205.
Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273–87.
Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–71.
Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology. 2015;29:235.
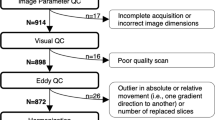
Roalf DR, Quarmley M, Elliott MA, Satterthwaite TD, Vandekar SN, Ruparel K, et al. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage. 2016;125:903–19.
Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55.
Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–78.
Graham MS, Drobnjak I, Zhang H. Realistic simulation of artefacts in diffusion MRI for validating post-processing correction techniques. Neuroimage. 2016;125:1079–94.
Zhang H, Yushkevich PA, Alexander DC, Gee JC. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Anal. 2006;10:764–85.
Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3.
Peters BD, Ikuta T, DeRosse P, John M, Burdick KE, Gruner P, et al. Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biol Psychiatry. 2014;75:248–56.
Wood SN Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc. 2004;99:673–86.
Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc. 2011;73:3–36.
Satterthwaite TD, Wolf DH, Calkins ME, Vandekar SN, Erus G, Ruparel K, et al. Structural brain abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry. 2016;73:515–24.
Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliott MA, Vandekar SN, et al. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci. 2014;111:8643–8.
Garcia de la Garza A, Vandekar S, Roalf DR, Ruparel K, Gur RC, Gur RE, et al. voxel: Mass-Univarite Voxelwise Analysis of Medical ImagingData. R package version132. 2017. https://rdrr.io/cran/voxel/
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97.
Bach M, Laun FB, Leemans A, Tax CM, Biessels GJ, Stieltjes B, et al. Methodological considerations on tract-based spatial statistics (TBSS). Neuroimage. 2014;100:358–69.
Shou H, Eloyan A, Lee S, Zipunnikov V, Crainiceanu A, Nebel M, et al. Quantifying the reliability of image replication studies: the image intraclass correlation coefficient (I2C2). Cogn Affect Behav Neurosci. 2013;13:714–24.
R-Core-Team. R: A Language and Enviroment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012.
Roalf DR, Gur RE, Verma R, Parker WA, Quarmley M, Ruparel K, et al. White matter microstructure in schizophrenia: associations to neurocognition and clinical symptomatology. Schizophr Res. 2015;161:42–49.
Beevor CE. On the course of the fibres of the cingulum and the posterior parts of the corpus callosum and fornix in the marmoset monkey. Philos Trans R Soc Lond B. 1891;182:135–99.
Brodal A. Neurological anatomy in relation to clinical medicine. USA: Oxford University Press; 1981.
Schmahmann JD, Pandya DN, Wang R, Dai G, D’arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–53.
Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–43.
Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–54.
Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208.
Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93:13–24.
Mighdoll MI, Tao R, Kleinman JE, Hyde TM. Myelin, myelin-related disorders, and psychosis. Schizophr Res. 2015;161:85–93.
Newcombe J, Uddin A, Dove R, Patel B, Turski L, Nishizawa Y, et al. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol. 2008;18:52–61.
Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol. 2001;50:169–80.
Schmidt A, Lenz C, Smieskova R, Harrisberger F, Walter A, Riecher-Rössler A, et al. Brain diffusion changes in emerging psychosis and the impact of state-dependent psychopathology. Neurosignals. 2015;23:71–83.
Thomas C, Moya L, Avidan G, Humphreys K, Jung KJ, Peterson MA, et al. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. J Cogn Neurosci. 2008;20:268–84.
Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: what have we learned? J Psychiatr Res. 2010;44:993–1004.
De Weijer A, Mandl R, Diederen K, Neggers S, Kahn R, Pol HH, et al. Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophr Res. 2011;130:68–77.
Ublinskii M, Semenova N, Lukovkina O, Sidorin S, Lebedeva I, Kaleda V, et al. Characteristics of diffusion in the corticospinal tract of patients with early stage of schizophrenia: diffusion tensor magnetic resonance imaging. Bull Exp Biol Med. 2015;159:29.
Acknowledgments
Thanks to the acquisition and recruitment team: Karthik Prabhakaran, Jeff Valdez, Raphael Gerraty, Marisa Riley, Jack Keefe, Elliott Yodh, Jason Blake, Prayosha Villa, R. Sean Gallagher and Rosetta Chiavacci.
Funding
This work was supported by the National Institute of Mental Health grants MH089983, MH089924, and MH087626. Additional support was provided by K01MH102609 to DRR; K23MH098130 and R01 MH107703 to TDS; R01 MH112847 to TDS and RTS; the Dowshen Program for Neuroscience at the University of Pennsylvania; and the Life Span Brain Institute (LiBI)—a collaboration between the University of Pennsylvania School of Medicine and Children’s Hospital of Philadelphia. This work was also supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. The funding sources were not directly involved in study design, collection, data analysis or interpretation, nor manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R.C.G. received royalties from the Brain Resource Centre. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Roalf, D.R., de la Garza, A.G., Rosen, A. et al. Alterations in white matter microstructure in individuals at persistent risk for psychosis. Mol Psychiatry 25, 2441–2454 (2020). https://doi.org/10.1038/s41380-019-0360-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0360-1
This article is cited by
-
Baseline symptom-related white matter tracts predict individualized treatment response to 12-week antipsychotic monotherapies in first-episode schizophrenia
Translational Psychiatry (2024)
-
Development of Brain Behavior Integration Systems Related to Criminal Culpability from Childhood to Young Adulthood: Does it Stop at 18 Years?
Journal of Pediatric Neuropsychology (2021)