Abstract
The complement system is a set of immune proteins involved in first-line defense against pathogens and removal of waste materials. Recent evidence has implicated the complement cascade in diseases involving the central nervous system, including schizophrenia. Here, we provide an up-to-date narrative review and critique of the literature on the relationship between schizophrenia and complement gene polymorphisms, gene expression, protein concentration, and pathway activity. A literature search identified 23 new studies since the first review on this topic in 2008. Overall complement pathway activity appears to be elevated in schizophrenia. Recent studies have identified complement component 4 (C4) and CUB and Sushi Multiple Domains 1 (CSMD1) as potential genetic markers of schizophrenia. In particular, there is some evidence of higher rates of C4B/C4S deficiency, reduced peripheral C4B concentration, and elevated brain C4A mRNA expression in schizophrenia patients compared to controls. To better elucidate the additive effects of multiple complement genotypes, we also conducted gene- and gene-set analysis through MAGMA which supported the role of Human Leukocyte Antigen class (HLA) III genes and, to a lesser extent, CSMD1 in schizophrenia; however, the HLA-schizophrenia association was likely driven by the C4 gene. Lastly, we identified several limitations of the literature on the complement system and schizophrenia, including: small sample sizes, inconsistent methodologies, limited measurements of neural concentrations of complement proteins, little exploration of the link between complement and schizophrenia phenotype, and lack of studies exploring schizophrenia treatment response. Overall, recent findings highlight complement components-in particular, C4 and CSMD1—as potential novel drug targets in schizophrenia. Given the growing availability of complement-targeted therapies, future clinical studies evaluating their efficacy in schizophrenia hold the potential to accelerate treatment advances.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Publishing; 2013.
Benros ME, Mortensen PB, Eaton WW. Autoimmune diseases and infections as risk factors for schizophrenia. Ann N Y Acad Sci. 2012;1262:56–66.
Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303–10.
Eaton WW, Byrne M, Ewald H, Mors O, Chen C-Y, Agerbo E, et al. Association of Schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry. 2006;163:521–8.
Khandaker GM, Zimbron J, Dalman C, Lewis G, Jones PB. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr Res. 2012;139:161–8.
Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013;43:239–57.
Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71.
Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2014;155:101–8.
Fernandes BS, Steiner J, Bernstein H-G, Dodd S, Pasco JA, Dean OM, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry. 2016;21:554–64.
Howes OD, McCutcheon R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry. 2017;7:e1024. 07
International Schizophrenia Consortium, Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52.
Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–7.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Irish Schizophrenia Genomics Consortium and the Wellcome Trust Case Control Consortium 2. Genome-wide association study implicates HLA-C*01:02 as a risk factor at the major histocompatibility complex locus in schizophrenia. Biol Psychiatry. 2012;72:620–8.
Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kähler AK, Akterin S, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–9.
Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–76.
Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–7.
Pouget JG, Gonçalves VF, Schizophrenia Working Group of the Psychiatric Genomics Consortium, Spain SL, Finucane HK, Raychaudhuri S, et al. Genome-wide association studies suggest limited immune gene enrichment in Schizophrenia compared to 5 autoimmune diseases. Schizophr Bull. 2016;42:1176–84.
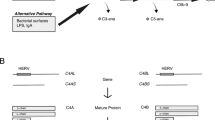
Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83.
Escudero-Esparza A, Kalchishkova N, Kurbasic E, Jiang WG, Blom AM. The novel complement inhibitor human CUB and Sushi multiple domains 1 (CSMD1) protein promotes factor I-mediated degradation of C4b and C3b and inhibits the membrane attack complex assembly. FASEB J Publ Fed Am Soc Exp Biol. 2013;27:5083–93.
Mayilyan KR, Weinberger DR, Sim RB. The complement system in Schizophrenia. Drug News Perspect. 2008;21:200–10.
Arakelyan A, Zakharyan R, Khoyetsyan A, Poghosyan D, Aroutiounian R, Mrazek F, et al. Functional characterization of the complement receptor type 1 and its circulating ligands in patients with schizophrenia. BMC Clin Pathol. 2011;11:10.
Soria L, dos S, Gubert C, de M, Cereser KM, Gama CS, Kapczinski F. Increased serum levels of C3 and C4 in patients with schizophrenia compared to eutymic patients with bipolar disorder and healthy controls. Rev Bras Psiquiatr. 2012;34:119–20.
Boyajyan A, Khoyetsyan A, Chavushyan A. Alternative complement pathway in schizophrenia. Neurochem Res. 2010;35:894–8.
Nimgaonkar VL, Prasad KM, Chowdari KV, Severance EG, Yolken RH. The complement system: a gateway to gene–environment interactions in schizophrenia pathogenesis. Mol Psychiatry. 2017;22:1554.
Charles A, Janeway J, Travers P, Walport M, Shlomchik MJ The complement system and innate immunity. In: Immunobiology: The Immune System in Health and Disease. 5th ed. New York: Garland Science; 2001.
Sarma JV, Ward PA. The Complement System. Cell Tissue Res. 2011;343:227–35.
Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–97.
Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, Lambris JD. Novel mechanisms and functions of complement. Nat Immunol. 2017;18:1288–98.
Ahearn JM, Fearon DT. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21). Adv Immunol. 1989;46:183–219.
Rodríguez de Córdoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sánchez-Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Mol Immunol. 2004;41:355–67.
Lachmann PJ, Müller-Eberhard HJ. The demonstration in human serum of “conglutinogen-activating factor” and its effect on the third component of complement. J Immunol Balt Md 1950. 1968;100:691–8.
Kraus DM, Elliott GS, Chute H, Horan T, Pfenninger KH, Sanford SD, et al. CSMD1 is a novel multiple domain complement-regulatory protein highly expressed in the central nervous system and epithelial tissues. J Immunol. 2006;176:4419–30.
Gorelik A, Sapir T, Haffner-Krausz R, Olender T, Woodruff TM, Reiner O. Developmental activities of the complement pathway in migrating neurons. Nat Commun. 2017;8:15096.
Niculescu T, Weerth S, Niculescu F, Cudrici C, Rus V, Raine CS, et al. Effects of complement C5 on apoptosis in experimental autoimmune encephalomyelitis. J Immunol Balt 1950. 2004;172:5702–6.
van Beek J, Nicole O, Ali C, Ischenko A, MacKenzie ET, Buisson A, et al. Complement anaphylatoxin C3a is selectively protective against NMDA-induced neuronal cell death. Neuroreport. 2001;12:289–93.
Cudrici C, Niculescu F, Jensen T, Zafranskaia E, Fosbrink M, Rus V, et al. C5b-9 terminal complex protects oligodendrocytes from apoptotic cell death by inhibiting caspase-8 processing and up-regulating FLIP. J Immunol Balt 1950. 2006;176:3173–80.
Soane L, Cho HJ, Niculescu F, Rus H, Shin ML. C5b-9 terminal complement complex protects oligodendrocytes from death by regulating Bad through phosphatidylinositol 3-kinase/Akt pathway. J Immunol Balt 1950. 2001;167:2305–11.
Zwaka TP, Torzewski J, Hoeflich A, Déjosez M, Kaiser S, Hombach V, et al. The terminal complement complex inhibits apoptosis in vascular smooth muscle cells by activating an autocrine IGF-1 loop. FASEB J Publ Fed Am Soc Exp Biol. 2003;17:1346–8.
Stokowska A, Atkins AL, Morán J, Pekny T, Bulmer L, Pascoe MC, et al. Complement peptide C3a stimulates neural plasticity after experimental brain ischaemia. Brain. J Neurol. 2017;140:353–69.
Shinjyo N, de Pablo Y, Pekny M, Pekna M. Complement peptide C3a promotes astrocyte survival in response to ischemic stress. Mol Neurobiol. 2016;53:3076–87.
Weerth SH, Rus H, Shin ML, Raine CS. Complement C5 in experimental autoimmune encephalomyelitis (EAE) facilitates remyelination and prevents gliosis. Am J Pathol. 2003;163:1069–80.
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78.
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705.
Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol. 2011;48:1592–603.
Chung EK, Yang Y, Rennebohm RM, Lokki M-L, Higgins GC, Jones KN, et al. Genetic sophistication of human complement components C4A and C4B and RP-C4-CYP21-TNX (RCCX) modules in the major histocompatibility complex. Am J Hum Genet. 2002;71:823–37.
Mack M, Bender K, Schneider PM. Detection of retroviral antisense transcripts and promoter activity of the HERV-K(C4) insertion in the MHC class III region. Immunogenetics. 2004;56:321–32.
Mayilyan KR, Wu YL, Kolachana B, McBride B, Yu CY, Weinberger DR. Lack of C4-short genes as a possible genetic mechanism of complement C4B protein level reductions in schizophrenia. Schizophr Bull. 2013;39:S104. Suppl 1.
Mayilyan KR, Weinberger DR, Wu YL, Kolachana B, McBride K, Yung CY Association of complement C4B gene deficiency with schizophrenia: Studies of European American families and controls. In: XV World Congress on Psychiatric Genetics 2007. p. 7–11.
Rudduck C, Beckman L, Franzén G, Jacobsson L, Lindström L. Complement factor C4 in schizophrenia. Hum Hered. 1985;35:223–6.
Wouters D, Van PS, Van A, der H, De MB, Schooneman D, Kuijpers TW, et al. High-throughput analysis of the C4 polymorphism by a combination of MLPA and isotype-specific ELISA’s. Mol Immunol. 2009;46:592–600.
Schroers R, Nöthen MM, Rietschel M, Albus M, Maier W, Schwab S, et al. Investigation of complement C4B deficiency in schizophrenia. Hum Hered. 1997;47:279–82.
Mayilyan KR, Dodds AW, Boyajyan AS, Soghoyan AF, Sim RB. Complement C4B protein in schizophrenia. World J Biol Psychiatry. 2008;9:225–30.
Mayilyan KR, Arnold JN, Presanis JS, Soghoyan AF, Sim RB. Increased complement classical and mannan-binding lectin pathway activities in schizophrenia. Neurosci Lett. 2006;404:336–41.
Shcherbakova I, Neshkova E, Dotsenko V, Platonova T, Shcherbakova E, Yarovaya G. The possible role of plasma kallikrein-kinin system and leukocyte elastase in pathogenesis of schizophrenia. Immunopharmacology. 1999;43:273–9.
Faludi G, Mirnics K. Synaptic changes in the brain of subjects with schizophrenia. Int J Dev Neurosci. 2011;29:305–9.
Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107.
Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73.
Liu W, Liu F, Xu X, Bai Y. Replicated association between the European GWAS locus rs10503253 at CSMD1 and schizophrenia in Asian population. Neurosci Lett. 2017;647:122–8.
Donohoe G, Walters J, Hargreaves A, Rose EJ, Morris DW, Fahey C, et al. Neuropsychological effects of the CSMD1 genome-wide associated schizophrenia risk variant rs10503253. Genes Brain Behav. 2013;12:203–9.
Koiliari E, Roussos P, Pasparakis E, Lencz T, Malhotra A, Siever LJ, et al. The CSMD1 genome-wide associated schizophrenia risk variant rs10503253 affects general cognitive ability and executive function in healthy males. Schizophr Res. 2014;154:42–7.
Rose EJ, Morris DW, Hargreaves A, Fahey C, Greene C, Garavan H, et al. Neural effects of the CSMD1 genome-wide associated schizophrenia risk variantrs10503253. Am J Med Genet Part B Neuropsychiatr Genet Publ Int SocPsychiatr Genet. 2013;162B:530–7.
Sakamoto S, Takaki M, Okahisa Y, Mizuki Y, Inagaki M, Ujike H, et al. Individual risk alleles of susceptibility to schizophrenia are associated with poor clinical and social outcomes. J Hum Genet. 2016;61:329–34.
Athanasiu L, Giddaluru S, Fernandes C, Christoforou A, Reinvang I, Lundervold AJ, et al. A genetic association study of CSMD1 and CSMD2 with cognitive function. Brain Behav Immun. 2017;61:209–16.
Xu W, Cohen-Woods S, Chen Q, Noor A, Knight J, Hosang G, et al. Genome-wide association study of bipolar disorder in Canadian and UK populations corroborates disease loci including SYNE1 and CSMD1. BMC Med Genet. 2014;15:2.
Cukier HN, Dueker ND, Slifer SH, Lee JM, Whitehead PL, Lalanne E, et al. Exome sequencing of extended families with autism reveals genes shared across neurodevelopmental and neuropsychiatric disorders. Mol Autism. 2014;5:1.
Sherva R, Wang Q, Kranzler H, Zhao H, Koesterer R, Herman A, et al. Genome-wide Association Study of Cannabis Dependence Severity, Novel Risk Variants, and Shared Genetic Risks. JAMA Psychiatry. 2016;73:472–80.
Meda SA, Ruaño G, Windemuth A, O’Neil K, Berwise C, Dunn SM, et al. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci. 2014;111:E2066–75.
Giddaluru S, Espeseth T, Salami A, Westlye LT, Lundquist A, Christoforou A, et al. Genetics of structural connectivity and information processing in the brain. Brain Struct Funct. 2016;221:4643–61.
Steen VM, Nepal C, Ersland KM, Holdhus R, Nævdal M, Ratvik SM, et al. Neuropsychological deficits in mice depleted of the schizophrenia susceptibility gene CSMD1. PloS One. 2013;8:e79501.
Roussos P, Katsel P, Davis KL, Siever LJ, Haroutunian V. A System-level transcriptomic analysis of schizophrenia using postmortem brain tissue samples. Arch Gen Psychiatry. 2012;69:1205–13.
GTEx Consortium. The genotype-tissue expression (GTEx) project. Nat Genet. 2013;45:580–5.
de Leeuw CA, Mooij JM, Heskes T, Posthuma D MAGMA: generalized gene-set analysis of GWAS Data. Tang H, editor. PLOS Comput Biol 2015;17;11:e1004219.
Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–40.
Fromer M, Roussos P, Sieberts SK, Johnson JS, Kavanagh DH, Perumal TM, et al. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci. 2016;19:1442–53.
Pérez-Santiago J, Diez-Alarcia R, Callado LF, Zhang JX, Chana G, White CH, et al. A combined analysis of microarray gene expression studies of the human prefrontal cortex identifies genes implicated in schizophrenia. J Psychiatr Res. 2012;46:1464–74.
Birnbaum R, Jaffe AE, Chen Q, Shin JH, Consortium B, Schubert CR, et al. Investigating the neuroimmunogenic architecture of schizophrenia. Mol Psychiatry. 2018;23:1251–60.
Beasley C, Shao L. 136. Increased expression of early complement components in frontal cortex in Schizophrenia. Schizophr Bull. 2017;43:S73–S73. suppl_1.
Ali FT, Abd El-Azeem EM, Hamed MA, Ali MAM, Abd Al-Kader NM, Hassan EA. Redox dysregulation, immuno-inflammatory alterations and genetic variants of BDNF and MMP-9 in schizophrenia: Pathophysiological and phenotypic implications. Schizophr Res. 2017;188:98–109.
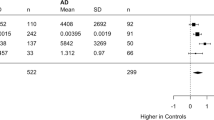
Fernandes BS, Cereser KM, Zortea K, Fries GR, Colpo G, Moreira L, et al. Complement system in bipolar disorders and schizophrenia: C3 and C4. Bipolar Disord. 2010;12:18–9.
Maes M, Delange J, Ranjan R, Meltzer HY, Desnyder R, Cooremans W, et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res. 1997;66:1–11.
Cazzullo CL, Saresella M, Roda K, Calvo MG, Bertrando P, Doria S, et al. Increased levels of CD8+ and CD4+ 45RA+ lymphocytes in schizophrenic patients. Schizophr Res. 1998;31:49–55.
Spivak B, Radwan M, Elimelech D, Baruch Y, Avidan G, Tyano S. A study of the complement system in psychiatric patients. Biol Psychiatry. 1989;26:640–2.
Spivak B, Radwan M, Brandon J, Baruch Y, Stawski M, Tyano S, et al. Reduced total complement haemolytic activity in schizophrenic patients. Psychol Med. 1993;23:315–8.
Wong CT, Tsoi WF, Saha N. Acute phase proteins in male Chinese schizophrenic patients in Singapore. Schizophr Res. 1996;22:165–71.
Li H, Zhang Q, Li N, Wang F, Xiang H, Zhang Z, et al. Plasma levels of Th17-related cytokines and complement C3 correlated with aggressive behavior in patients with schizophrenia. Psychiatry Res. 2016;246:700–6.
Idonije OB, Akinlade KS, Ihenyen O, Arinola OG. Complement factors in newly diagnosed Nigerian schizoprenic patients and those on antipsychotic therapy. Niger J Physiol Sci Publ Physiol Soc Niger. 2012;27:19–21.
Morera AL, Henry M, Garcia-Hernandez A, Fernandez-Lopez L. Acute phase proteins as biological markers of negative psychopathology in paranoid schizophrenia. Actas Esp Psiquiatr. 2007;35:249–52.
Laskaris L, Chana G, Weickert CS, Bousman C, Baune B, McGorry P, et al. Increased C3 and C4 proteins in serum of FEP and UHR patients: implications for inflammatory subtyping in SCZ. Biol Psychiatry. 2017;81:S27–8.
Boyajyan A, Khoyetsyan A, Tsakanova G, Sim RB. Cryoglobulins as indicators of upregulated immune response in schizophrenia. Clin Biochem. 2008;41:355–60.
Severance EG, Gressitt KL, Halling M, Stallings CR, Origoni AE, Vaughan C, et al. Complement C1q formation of immune complexes with milk caseins and wheat glutens in schizophrenia. Neurobiol Dis. 2012;48:447–53.
Kirschfink M, Mollnes TE. Modern complement analysis. Clin Diagn Lab Immunol. 2003;10:982–9.
Palarasah Y, Nielsen C, Sprogøe U, Christensen ML, Lillevang S, Madsen HO, et al. Novel assays to assess the functional capacity of the classical, the alternative and the lectin pathways of the complement system. Clin Exp Immunol. 2011;164:388–95.
Li Y, Zhou K, Zhang Z, Sun L, Yang J, Zhang M, et al. Label-free quantitative proteomic analysis reveals dysfunction of complement pathway in peripheral blood of schizophrenia patients: evidence for the immune hypothesis of schizophrenia. Mol Biosyst. 2012;8:2664–71.
Zhang C, Zhang Y, Cai J, Chen M, Song L. Complement 3 and metabolic syndrome induced by clozapine: a cross-sectional study and retrospective cohort analysis. Pharm J. 2017;17:92–7.
Shcherbakova IV, Neshkova EA, Dotsenko VL, Kozlov LV, Mishin AA, Platonova TP, et al. [Activation of kallikrein-kinin system, degranulating activity of neutrophils and blood-brain barrier in schizophrenia]. Zh Nevrol Psikhiatr Im S S Korsakova. 1998;98:38–41.
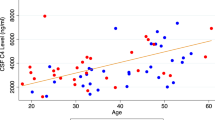
Hu WT, Watts KD, Tailor P, Nguyen TP, Howell JC, Lee RC, et al. CSF complement 3 and factor H are staging biomarkers in Alzheimer’s disease. Acta Neuropathol Commun. 2016 Feb 17;4:14.
Lally J, Gaughran F, Timms P, Curran SR. Treatment-resistant schizophrenia: current insights on the pharmacogenomics of antipsychotics. Pharm Pers Med. 2016;9:117–29.
Li J, Loebel A, Meltzer HY. Identifying the genetic risk factors for treatment response to lurasidone by genome-wide association study: a meta-analysis of samples from three independent clinical trials. Schizophr Res. 2018;199:203–13.
Fond G, d’Albis M-A, Jamain S, Tamouza R, Arango C, Fleischhacker WW, et al. The Promise of Biological Markers for Treatment Response in First-Episode Psychosis: A Systematic Review. Schizophr Bull. 2015;41:559–73.
Girardin FR, Poncet A, Perrier A, Vernaz N, Pletscher M, Samer CF, et al. Cost-effectiveness of HLA-DQB1/HLA-B pharmacogenetic-guided treatment and blood monitoring in US patients taking clozapine. Pharmacogenomics J. 2019;19:211–8.
MacDonald ML, Alhassan J, Newman JT, Richard M, Gu H, Kelly RM, et al. Selective loss of smaller spines in Schizophrenia. Am J Psychiatry. 2017;174:586–94.
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 29 Feb 2000. Identifier NCT02605993, Open-label, Multiple Ascending Dose Study of ALXN1210 in Patients With Paroxysmal Nocturnal Hemoglobinuria; 2015 Nov [cited 2019 Jan 23]. Available from: https://clinicaltrials.gov/ct2/show/NCT02605993.
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 29 Feb 2000. Identifier NCT02763644, Efficacy and Safety of LFG316 in Transplant Associated Microangiopathy (TAM) Patients; 2016 May 5 [cited 2019 Jan 23]. Available froom: https://clinicaltrials.gov/ct2/show/NCT02763644.
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 29 Feb 2000 Feb. Identifier NCT02515942, CLG561 Proof-of-Concept Study as a Monotherapy and in Combination With LFG316 in Subjects With Geographic Atrophy (GA); 2015 Aug 5 [cited 2019 Jan 23]. Available from: https://clinicaltrials.gov/ct2/show/NCT02515942.
National Center for Biotechnology Information (NCBI) [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [1988] - [cited 2018 Jun 5]. Available from: https://www.ncbi.nlm.nih.gov/gene.
Kano S, Nwulia E, Niwa M, Chen Y, Sawa A, Cascella N. Altered MHC class I expression in dorsolateral prefrontal cortex of nonsmoker patients with schizophrenia. Neurosci Res. 2011;71:289–93.
Ni J, Hu S, Zhang J, Tang W, Lu W, Zhang C. A preliminary genetic analysis of complement 3 gene and Schizophrenia. PloS One. 2015;10:e0136372.
Zhang C, Lv Q, Fan W, Tang W, Yi Z. Influence of CFH gene on symptom severity of schizophrenia. Neuropsychiatr Dis Treat. 2017;13:697–706.
Kucharska-Mazur J, Tarnowski M, Dołęgowska B, Budkowska M, Pędziwiatr D, Jabłoński M, et al. Novel evidence for enhanced stem cell trafficking in antipsychotic-naïve subjects during their first psychotic episode. J Psychiatr Res. 2014;49:18–24.
Foldager L, Steffensen R, Thiel S, Als TD, Nielsen HJ, Nordentoft M, et al. MBL and MASP-2 concentrations in serum and MBL2 promoter polymorphisms are associated to schizophrenia. Acta Neuropsychiatr. 2012;24:199–207.
Acknowledgements
This study was funded by the Ontario Ministry of Research, Innovation, and Science and University of Toronto’s Faculty of Medicine. We would also like to thank Larry and Judy Tanenbaum for their generous donations to the Centre for Addiction and Mental Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Woo, J.J., Pouget, J.G., Zai, C.C. et al. The complement system in schizophrenia: where are we now and what’s next?. Mol Psychiatry 25, 114–130 (2020). https://doi.org/10.1038/s41380-019-0479-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0479-0
This article is cited by
-
Association study of the complement component C4 gene and suicide risk in schizophrenia
Schizophrenia (2024)
-
Common risk alleles for schizophrenia within the major histocompatibility complex predict white matter microstructure
Translational Psychiatry (2024)
-
Molecular mapping of a core transcriptional signature of microglia-specific genes in schizophrenia
Translational Psychiatry (2023)
-
Association of elevated levels of peripheral complement components with cortical thinning and impaired logical memory in drug-naïve patients with first-episode schizophrenia
Schizophrenia (2023)
-
Schizophrenia and psychedelic state: Dysconnection versus hyper-connection. A perspective on two different models of psychosis stemming from dysfunctional integration processes
Molecular Psychiatry (2023)