Abstract
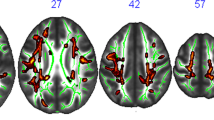
The metabolic serum marker HbA1c has been associated with both impaired cognitive performance and altered white matter integrity in patients suffering from diabetes mellitus. However, it remains unclear if higher levels of HbA1c might also affect brain structure and function in healthy subjects. With the present study we therefore aimed to investigate the relationship between HbA1c levels and cognitive performance as well as white matter microstructure in healthy, young adults. To address this question, associations between HbA1c and cognitive measures (NIH Cognition Toolbox) as well as DTI-derived imaging measures of white matter microstructure were investigated in a publicly available sample of healthy, young adults as part of the Humane Connectome Project (n = 1206, mean age = 28.8 years, 45.5% male). We found that HbA1c levels (range 4.1–6.3%) were significantly inversely correlated with measures of cognitive performance. Higher HbA1c levels were associated with significant and widespread reductions in fractional anisotropy (FA) controlling for age, sex, body mass index, ethnicity, and education. FA reductions were furthermore found to covary with measures of cognitive performance. The same pattern of results could be observed in analyses restricted to participants with HBA1c levels below 5.7%. The present study demonstrates that low-grade HbA1c variation below diagnostic threshold for diabetes is related to both cognitive performance and white matter integrity in healthy, young adults. These findings highlight the detrimental impact of metabolic risk factors on brain physiology and underscore the importance of intensified preventive measures independent of the currently applied diagnostic HbA1c cutoff scores.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
13 March 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41380-020-0710-z
References
World Health Organization. Global report on diabetes. Geneva: World Health Organization; 2016.
World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Diabetes research and clinical practice. Geneva: World Health Organization; 2011.
American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S13–S27.
Seaquist ER. The final frontier: how does diabetes affect the brain? Diabetes. 2010;59:4–5.
Ott A, Stolk RP, Hofman A, Van Harskamp F, Grobbee DE, Breteler MMB. Association of diabetes mellitus and dementia: the Rotterdam study. Diabetologia. 1996;39:1392–7.
Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3:75–89.
Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr. Rev. 2008;29:494–511.
Fox LA, Hershey T, Mauras N, Arbeláez AM, Tamborlane WV, Buckingham B, et al. Persistence of abnormalities in white matter in children with type 1 diabetes. Diabetologia. 2018;61:1538–47.
Barnea-Goraly N, Raman M, Mazaika P, Marzelli M, Hershey T, Weinzimer SA, et al. Alterations in white matter structure in young children with type 1 diabetes. Diabetes Care. 2014;37:332–40.
Van Duinkerken E, Schoonheim MM, IJzerman RG, Klein M, Ryan CM, Moll AC, et al. Diffusion tensor imaging in type 1 diabetes: decreased white matter integrity relates to cognitive functions. Diabetologia. 2012;55:1218–20.
Kochunov P, Coyle T, Lancaster J, Robin DA, Hardies J, Kochunov V, et al. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage. 2010;49:1190–9.
Bennett IJ, Madden DJ. Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2014;276:187–205.
Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31:1554–62.
Muetzel RL, Collins PF, Mueller BA, M. Schissel A, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. Neuroimage 2008;39:1918–25.
Kochunov P, Robin DA, Royall DR, Coyle T, Lancaster J, Kochunov V, et al. Can structural MRI indices of cerebral integrity track cognitive trends in executive control function during normal maturation and adulthood? Hum Brain Mapp. 2009;30:2581–94.
Konrad A, Vucurevic G, Musso F, Stoeter P, Winterer G. Correlation of brain white matter diffusion anisotropy and mean diffusivity with reaction time in an oddball task. Neuropsychobiology. 2009;60:55–66.
McLaren L. Socioeconomic status and obesity. Epidemiol. Rev. 2007;29:29–48.
Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ et al. Cognition assessment using the NIH Toolbox. Neurology 2013;80:S49–S53.
Galobardes B, Shaw M, Lawlor DA, Lynch JW, Smith GD. Indicators of socioeconomic position (part 1). J. Epidemiol. Community Health. 2006;60:7–12.
Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K. The WU-Minn human connectome project: an overview. Neuroimage. 2013;80:62–79.
Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TEJ, Bucholz R, et al. The human connectome project: a data acquisition perspective. NeuroImage. 2012;62:2222–31.
Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80:S2–6.
Heaton RK, Akshoomoff N, Tulsky D, Mungas D, Weintraub S, Dikmen S, et al. Reliability and validity of composite scores from the NIH toolbox cognition battery in adults. J Int Neuropsychol Soc. 2014;20:588–98.
Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, et al. Multiplexed echo planar imaging for sub-second whole brain fmri and fast diffusion imaging. PLoS One. 2010;5:e15710.
Sotiropoulos SN, Moeller S, Jbabdi S, Xu J, Andersson JL, Auerbach EJ, et al. Effects of image reconstruction on fiber orientation mapping from multichannel diffusion MRI: reducing the noise floor using SENSE. Magn Reson Med. 2013;70:1682–9.
Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 2013;80:105–24.
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505.
Repple J, Opel N, Meinert S, Redlich R, Hahn T, Winter NR, et al. Elevated body-mass index is associated with reduced white matter integrity in two large independent cohorts. Psychoneuroendocrinology. 2018;91:179–85.
Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98.
Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–47.
Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, et al. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–87.
Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22:1979–92.
Opel N, Redlich R, Kaehler C, Grotegerd D, Dohm K, Heindel W, et al. Prefrontal gray matter volume mediates genetic risks for obesity. Mol Psychiatry. 2017;22:703–10. http://www.ncbi.nlm.nih.gov/pubmed/28348383.
Opel N, Redlich R, Dohm K, Zaremba D, Goltermann J, Repple J, et al. Mediation of the influence of childhood maltreatment on depression relapse by cortical structure: a 2-year longitudinal observational study. The Lancet Psychiatry 2019. https://doi.org/10.1016/S2215-0366(19)30044-6.
Mackey S, Chaarani B, Kan KJ, Spechler PA, Orr C, Banaschewski T, et al. Brain Regions Related to Impulsivity Mediate the Effects of Early Adversity on Antisocial Behavior. Biol Psychiatry 2017. https://doi.org/10.1016/j.biopsych.2015.12.027.
Alfaro FJ, Gavrieli A, Saade-Lemus P, Lioutas VA, Upadhyay J, Novak V. White matter microstructure and cognitive decline in metabolic syndrome: a review of diffusion tensor imaging. Metabolism. 2018;78:52–68.
Pappas C, Andel R, Infurna FJ, Seetharaman S. Glycated haemoglobin (HbA1c), diabetes and trajectories of change in episodic memory performance. J Epidemiol Community Health. 2017;71:115–20.
Ravona-Springer R, Moshier E, Schmeidler J, Godbold J, Akrivos J, Rapp M, et al. Changes in glycemic control are associated with changes in cognition in non-diabetic elderly. J Alzheimer’s Dis. 2012;30:299–309.
Malone JI, Hanna S, Saporta S, Mervis RF, Park CR, Chong L, et al. Hyperglycemia not hypoglycemia alters neuronal dendrites and impairs spatial memory. Pediatr Diabetes. 2008;9:531–9.
Hernández-Fonseca JP, Rincón J, Pedreañez A, Viera N, Arcaya JL, Carrizo E, et al. Structural and ultrastructural analysis of cerebral cortex, cerebellum, and hypothalamus from diabetic rats. Exp Diabetes. Res 2009;2009:1–12.
Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012. 363–74.
Wang X, Bao W, Liu J, OuYang Y-Y, Wang D, Rong S, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36:166–75.
Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3.
Frodl T, Carballedo A, Hughes MM, Saleh K, Fagan A, Skokauskas N, et al. Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl Psychiatry. http://www.ncbi.nlm.nih.gov/pubmed/22832853.
van Velzen LS, Schmaal L, Milaneschi Y, van Tol M-J, van der Wee NJA, Veltman DJ, et al. Immunometabolic dysregulation is associated with reduced cortical thickness of the anterior cingulate cortex. Brain Behav Immun. 2017;60:361–8. http://www.ncbi.nlm.nih.gov/pubmed/27989860.
Benedetti F, Poletti S, Hoogenboezem TA, Mazza E, Ambrée O, de Wit H, et al. Inflammatory cytokines influence measures of white matter integrity in bipolar disorder. J Affect Disord. 2016;202:1–9.
Poletti S, de Wit H, Mazza E, Wijkhuijs AJM, Locatelli C, Aggio V, et al. Th17 cells correlate positively to the structural and functional integrity of the brain in bipolar depression and healthy controls. Brain Behav Immun. 2017;61:317–25.
Racine AM, Merluzzi AP, Adluru N, Norton D, Koscik RL, Clark LR et al. Association of longitudinal white matter degeneration and cerebrospinal fluid biomarkers of neurodegeneration, inflammation and Alzheimer’s disease in late-middle-aged adults. Brain Imaging Behav 2019;13:41–52.
Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–15.
Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, et al. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2012;33:9–20.
Brito NH, Noble KG. Socioeconomic status and structural brain development. Front. Neurosci. 2014;8. https://doi.org/10.3389/fnins.2014.00276.
Pechey R, Monsivais P. Socioeconomic inequalities in the healthiness of food choices: exploring the contributions of food expenditures. Prev Med. 2016;88:203–9.
Acknowledgements
This work was funded by the German Research Foundation (DFG, grant FOR2107 DA1151/5–1 and DA1151/5–2 to UD; SFB-TRR58, Projects C09 and Z02 to UD) and the Interdisciplinary Center for Clinical Research (IZKF) of the medical faculty of Münster (grant Dan3/012/17 to UD). Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Funding
VA is a member of the advisory board of, or has given presentations on behalf of, the following companies: Astra-Zeneca, Janssen-Organon, Lilly, Lundbeck, Servier, Pfizer, Otsuka, and Trommsdorff. These affiliations are of no relevance to the work described in the paper. H. Kugel has received consultation fees from MR: comp GmbH, Testing Services for MR Safety.
Author information
Authors and Affiliations
Contributions
JR and GK made substantial contributions to the conception, design of the work, and drafted the work. SM, KF, DG, and JG made substantial contributions to the analysis of this work and the interpretation of data. VA, BB, and UD have substantially revised the work. NO made substantial contributions to the conception, design of the work, and drafted the work. All authors approved the submitted version and agreed both to be personally accountable for the author’s own contributions and that questions related to the accuracy or integrity of any part of the work are appropriately investigated.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Repple, J., Karliczek, G., Meinert, S. et al. Variation of HbA1c affects cognition and white matter microstructure in healthy, young adults. Mol Psychiatry 26, 1399–1408 (2021). https://doi.org/10.1038/s41380-019-0504-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0504-3
This article is cited by
-
Beyond BMI: cardiometabolic measures as predictors of impulsivity and white matter changes in adolescents
Brain Structure and Function (2023)
-
Association of brain white matter microstructure with cognitive performance in major depressive disorder and healthy controls: a diffusion-tensor imaging study
Molecular Psychiatry (2022)
-
Adipositas und Gehirngesundheit
Psychotherapeut (2021)
-
Severity of current depression and remission status are associated with structural connectome alterations in major depressive disorder
Molecular Psychiatry (2020)