Abstract
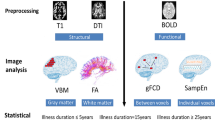
Several prominent theories of schizophrenia suggest that structural white matter pathologies may follow a developmental, maturational, and/or degenerative process. However, a lack of lifespan studies has precluded verification of these theories. Here, we analyze the largest sample of carefully harmonized diffusion MRI data to comprehensively characterize age-related white matter trajectories, as measured by fractional anisotropy (FA), across the course of schizophrenia. Our analysis comprises diffusion scans of 600 schizophrenia patients and 492 healthy controls at different illness stages and ages (14–65 years), which were gathered from 13 sites. We determined the pattern of age-related FA changes by cross-sectionally assessing the timing of the structural neuropathology associated with schizophrenia. Quadratic curves were used to model between-group FA differences across whole-brain white matter and fiber tracts at each age; fiber tracts were then clustered according to both the effect-sizes and pattern of lifespan white matter FA differences. In whole-brain white matter, FA was significantly lower across the lifespan (up to 7%; p < 0.0033) and reached peak maturation younger in patients (27 years) compared to controls (33 years). Additionally, three distinct patterns of neuropathology emerged when investigating white matter fiber tracts in patients: (1) developmental abnormalities in limbic fibers, (2) accelerated aging and abnormal maturation in long-range association fibers, (3) severe developmental abnormalities and accelerated aging in callosal fibers. Our findings strongly suggest that white matter in schizophrenia is affected across entire stages of the disease. Perhaps most strikingly, we show that white matter changes in schizophrenia involve dynamic interactions between neuropathological processes in a tract-specific manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Code availability
Our multi-site diffusion MRI harmonization software is available per request. Please request an access through e-mail: skarayumak@bwh.harvard.edu.
Notes
early onset, first episode, early course, chronic
Forceps major (posterior forceps), forceps minor (anterior forceps), for the rest left and right hemisphere separately: cingulum (cingulate gyrus portion (CING1) and hippocampal (CING2) portion separately), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), uncinate fasciculus (UF).
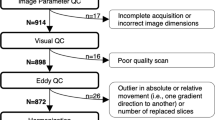
Bonferroni correction was performed to control for the number of fibers and whole-brain (n = 15).
References
Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363:2063–72.
Kubicki M, McCarley R, Westin C-F, Park H-J, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30.
Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52.
Menon RR, Barta PE, Aylward EH, Richards SS, Vaughn DD, Tien AY, et al. Posterior superior temporal gyrus in schizophrenia: grey matter changes and clinical correlates. Schizophr Res. 1995;16:127–35.
Schnack HG, van Haren NEM, Nieuwenhuis M, Hulshoff Pol HE, Cahn W, Kahn RS. Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. Am J Psychiatry. 2016;173:607–16.
Mitelman SA, Canfield EL, Newmark RE, Brickman AM, Torosjan Y, Chu K-W, et al. Longitudinal Assessment of Gray and White Matter in Chronic Schizophrenia: A Combined Diffusion-Tensor and Structural Magnetic Resonance Imaging Study. Open Neuroimag J. 2009;3:31–47.
Ohtani T, Bouix S, Hosokawa T, Saito Y, Eckbo R, Ballinger T, et al. Abnormalities in white matter connections between orbitofrontal cortex and anterior cingulate cortex and their associations with negative symptoms in schizophrenia: a DTI study. Schizophr Res. 2014;157:190–7.
Ohtani T, Bouix S, Lyall AE, Hosokawa T, Saito Y, Melonakos E, et al. Abnormal white matter connections between medial frontal regions predict symptoms in patients with first episode schizophrenia. Cortex. 2015;71:264–76.
Kochunov P, Hong LE. Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr Bull. 2014;40:721–8.
Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophr Res. 2016;176:83–94.
Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. J Psychiatr Res. 1999;33:513–21.
Xiao Y, Sun H, Shi S, Jiang D, Tao B, Zhao Y, et al. White matter abnormalities in never-treated Patients with long-term schizophrenia. Am J Psychiatry. 2018;175:1129–36.
McGrath JJ, Féron FP, Burne THJ, Mackay-Sim A, Eyles DW. The neurodevelopmental hypothesis of schizophrenia: a review of recent developments. Ann Med. 2003;35:86–93.
Murray RM, Castle D, O’Callaghan E, Lewis S. Classifying schizophrenia into neurodevelopmental and adult onset forms. Schizophr Res. 1992;6:170.
Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–48.
van Haren NEM, Hulshoff Pol HE, Schnack HG, Cahn W, Brans R, Carati I, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63:106–13.
French L, Gray C, Leonard G, Perron M, Pike GB, Richer L, et al. Early cannabis use, polygenic risk score for schizophrenia and brain maturation in adolescence. JAMA Psychiatry. 2015;72:1002–11.
Cropley VL, Klauser P, Lenroot RK, Bruggemann J, Sundram S, Bousman C, et al. Accelerated gray and white matter deterioration with age in schizophrenia. Am J Psychiatry. 2017;174:286–95.
Gama C. 175.4 The relationship of aging and inflammatory biomarkers to gray matter volume and episodic memory performance in schizophrenia: evidence of pathological accelerated aging. Schizophr Bull. 2017;43:S90.
Sexton CE, Walhovd KB, Storsve AB, Tamnes CK, Westlye LT, Johansen-Berg H, et al. Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. J Neurosci. 2014;34:15425–36.
Xiao Y, Sun H, Shi S, Jiang D, Tao B, Zhao Y, et al. White matter abnormalities in never-treated patients with long-term schizophrenia. Am J Psychiatry. 2018;175:1129–36. appiajp201817121402
Meng L, Li K, Li W, Xiao Y, Lui S, Sweeney JA, et al. Widespread white-matter microstructure integrity reduction in first-episode schizophrenia patients after acute antipsychotic treatment. Schizophr Res. 2018. https://doi.org/10.1016/j.schres.2018.08.021.
Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, et al. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8:153–82.
Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, et al. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA–DTI working group. NeuroImage. 2013;81:455–69.
Blokland GAM, del Re EC, Mesholam-Gately RI, Jovicich J, Trampush JW, Keshavan MS, et al. The Genetics of Endophenotypes of Neurofunction to Understand Schizophrenia (GENUS) consortium: a collaborative cognitive and neuroimaging genetics project. Schizophr Res. 2018;195:306–17.
Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67:578–88.
Cheng W, Palaniyappan L, Li M, Kendrick KM, Zhang J, Luo Q, et al. Addendum: Voxel-based, brain-wide association study of aberrant functional connectivity in schizophrenia implicates thalamocortical circuitry. NPJ Schizophr. 2018;4:19.
Loughland C, Draganic D, McCabe K, Richards J, Nasir A, Allen J, et al. Australian Schizophrenia Research Bank: a database of comprehensive clinical, endophenotypic and genetic data for aetiological studies of schizophrenia. Aust N Z J Psychiatry. 2010;44:1029–35.
Elliott LT, Sharp K, Alfaro-Almagro F, Shi S, Miller KL, Douaud G, et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature. 2018;562:210–6.
Tamminga CA, Pearlson G, Keshavan M, Sweeney J, Clementz B, Thaker G. Bipolar and schizophrenia network for intermediate phenotypes: outcomes across the psychosis continuum. Schizophr Bull. 2014;40:S131–7.
Cetin Karayumak S, Bouix S, Ning L, James A, Crow T, Shenton M, et al. Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters. Neuroimage. 2019;184:180–200.
Mirzaalian H, Ning L, Savadjiev P, Pasternak O, Bouix S, Michailovich O, et al. Inter-site and inter-scanner diffusion MRI data harmonization. Neuroimage. 2016;135:311–23.
Mirzaalian H, Ning L, Savadjiev P, Pasternak O, Bouix S, Michailovich O, et al. Multi-site harmonization of diffusion MRI data in a registration framework. Brain Imaging Behav. 2017;12:284–95.
Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, et al. Neuroimaging of the Philadelphia Neurodevelopmental Cohort. Neuroimage. 2014;86:544–53.
Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, et al. The Philadelphia Neurodevelopmental Cohort: a publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 2016;124:1115–9.
Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55.
Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–88.
Avants BB, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J, et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 2010;49:2457–66.
Varentsova A, Zhang S, Arfanakis K. Development of a high angular resolution diffusion imaging human brain template. Neuroimage. 2014;91:177–86.
Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–44.
Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–52.
Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nat Commun. 2014;5:4932.
Cox SR, Ritchie SJ, Tucker-Drob EM, Liewald DC, Hagenaars SP, Davies G, et al. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nat Commun. 2016;7:13629.
Tønnesen S, Kaufmann T, Doan NT, Alnæs D, Córdova-Palomera A, Meer D, et al. White matter aberrations and age-related trajectories in patients with schizophrenia and bipolar disorder revealed by diffusion tensor imaging. Sci Rep. 2018;8:14129.
Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–55.
Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–46.
Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL, et al. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain. 2010;133:1494–504.
Whitford TJ, Kubicki M, Schneiderman JS, O’Donnell LJ, King R, Alvarado JL, et al. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol Psychiatry. 2010;68:70–7.
Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–23.
Konopaske GT, Dorph-Petersen K-A, Sweet RA, Pierri JN, Zhang W, Sampson AR, et al. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63:759–65.
Kubicki M, Lyall AE. Antipsychotics and their impact on cerebral white matter: part of the problem or part of the solution? Am J Psychiatry. 2018;175:1056–7.
Kyriakopoulos M, Samartzis L, Dima D, Hayes D, Corrigall R, Barker G, et al. P03-111 - Does antipsychotic medication affect white matter in schizophrenia and bipolar disorder? a review of diffusion tensor imaging literature. Eur Psychiatry. 2011;26:1280.
Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23:1261–9.
Acknowledgements
We gratefully acknowledge funding provided by the following National Institutes of Health (NIH) grants: R01MH102377, K24MH110807 (PI: Dr. Marek Kubicki), R01MH119222 (PI: Dr. Yogesh Rathi), R03 MH110745, K01 MH115247–01A1 (PI: Dr. Amanda Lyall), VA Merit Award and U01 MH109977 (PI: Dr. Martha Shenton), R01MH108574 (PI: Dr. Pasternak), MRC G0500092 (PI: Dr. Anthony James), R01MH076995, P30MH090590, P50MH080173 (PI: Dr. Philip Szeszko), R01MH092440, R01MH078113 (PI: Dr. Matcheri Keshavan), R01MH077851 (PI: Dr. Carol Tamminga), R01MH077945 (PI: Dr. Godfrey Pearlson), R01MH077852 (PI: Dr. Gunvant Thaker), R01MH077862 (PI: Dr. John Sweeney). We also acknowledge funding provided by the Swiss National Science Foundation (SNF) grant 152619 (PI: Dr. Sebastian Walther) and National Research Foundation of Korea (NRF) grant NRF-2012R1A1A1006514) (PI: Dr. Jungsun Lee).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cetin-Karayumak, S., Di Biase, M.A., Chunga, N. et al. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol Psychiatry 25, 3208–3219 (2020). https://doi.org/10.1038/s41380-019-0509-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0509-y
This article is cited by
-
Changes in kynurenine metabolites in the gray and white matter of the dorsolateral prefrontal cortex of individuals affected by schizophrenia
Schizophrenia (2024)
-
N-acetylcysteine during critical neurodevelopmental periods prevents behavioral and neurochemical deficits in the Poly I:C rat model of schizophrenia
Translational Psychiatry (2024)
-
Harmonized diffusion MRI data and white matter measures from the Adolescent Brain Cognitive Development Study
Scientific Data (2024)
-
Association of homocysteine with white matter dysconnectivity in schizophrenia
Schizophrenia (2024)
-
Ameliorative effects of Fingolimod (FTY720) on microglial activation and psychosis-related behavior in short term cuprizone exposed mice
Molecular Brain (2023)