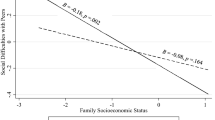
Abstract
The progression of lifelong trajectories of socioeconomic inequalities in health and mortality begins in childhood. Dysregulation in cortisol, a stress hormone that is the primary output of the hypothalamus–pituitary–adrenal (HPA) axis, has been hypothesized to be a mechanism for how early environmental adversity compromises health. However, despite the popularity of cortisol as a biomarker for stress and adversity, little is known about whether cortisol output differs in children being raised in socioeconomically disadvantaged environments. Here, we show that there are few differences between advantaged and disadvantaged children in their cortisol output. In 8–14-year-old children from the population-based Texas Twin Project, we measured cortisol output at three different timescales: (a) diurnal fluctuation in salivary cortisol (n = 400), (b) salivary cortisol reactivity and recovery after exposure to the Trier Social Stress Test (n = 444), and (c) cortisol concentration in hair (n = 1210). These measures converged on two moderately correlated, yet distinguishable, dimensions of HPA function. We tested differences in cortisol output across nine aspects of social disadvantage at the home (e.g., family socioeconomic status), school (e.g., average levels of academic achievement), and neighborhood (e.g., concentrated poverty). Children living in neighborhoods with higher concentrated poverty had higher diurnal cortisol output, as measured in saliva; otherwise, child cortisol output was unrelated to any other aspect of social disadvantage. Overall, we find limited support for alteration in HPA axis functioning as a general mechanism for the health consequences of socioeconomic inequality in childhood.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
While results, FIML summary data, analytic scripts, and generated outputs will be uploaded and instantly available for all researchers to use, our policy regarding the access of raw data files is separate. The data file related to this project contains particularly sensitive information, including each child’s geocoded neighborhood and school information and sensitive endocrinological data. To this end, researchers will be able to obtain the data file through managed access. Requests for managed access should be sent to EMT-D and KPH, joint principal investigators of the Texas Twin Project.
Code availability
Results, FIML summary data, analytic scripts, and generated outputs will be uploaded and instantly available for all researchers to use.
References
Marmot M, Davey-Smith G, Stansfeld S, Patel C, North F, Head J, et al. Health inequalities among British civil servants: teh Whitehall II study. Lancet. 1991;337:1387–93.
Chetty R, Stepner M, Abraham S, Lin S, Scuderi B, Turner N, et al. The association between income and life expectancy in the United States, 2001-2014. JAMA. 2016;315:1750–66.
Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci USA. 2015;112:1–6.
Saez E. Striking it richer: the evolution of top incomes in the United States (updated with 2014 preliminary estimates). Univ Calif Berkley Work Pap. 2015;2015:120–8.
Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–76.
Miller GE, Chen E. The biological residue of childhood poverty. Child Dev Perspect. 2013;7:67–73.
Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:240–51.
Hertzman C, Boyce T. How Experience gets under the skin to create gradients in developmental health. Annu Rev Public Health. 2010;31:329–47.
Mcewen BS. Correction for McEwen, brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci USA. 2013;110:2–1561.
McLaughlin KA, Lane RD, Bush NR. Introduction to the special issue of psychosomatic medicine: mechanisms linking early-life adversity to physical health. Psychosom Med. 2016;78:976–8.
Danese A. Commentary: biological embedding of childhood adversity: where do we go from here? A reflection on Koss and Gunnar (2018). J Child Psychol Psychiatry Allied Discip. 2018;59:347–9.
Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–92.
Meaney MJ. Mother nurture and the social definition of neurodevelopment. Proc Natl Acad Sci USA. 2016;113:6094–6.
Bhatnagar S, Meaney MJ. Hypothalamic‐pituitary‐adrenal function in chronic intermittently cold‐stressed neonatally handled and non handled rats. J Neuroendocrinol. 1995;7:97–108.
Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Sharma S, et al. Maternal care, hippocampal glucocorticoid responses to stress receptor (GR) expression in the hypothalamic-pituitary-adrenal response to stress. Science. 1997;277:1659–62.
Hellstrom IC, Dhir SK, Diorio JC, Meaney MJ. Maternal licking regulates hippocampal glucocorticoid receptor transcription through a thyroid hormone-serotonin-NGFI-A signalling cascade. Philos Trans R Soc B Biol Sci. 2012;367:2495–510.
Staufenbiel SM, Pennix BWJH, Spijker AT, Elzinga M, van Rossum EFC. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38:1220–35.
Kristenson M, Garvin P, Lundberg U. Psychosocial work stressors and salivary. In: The role of saliva cortisol measurement in health and disease. Danvers, MA: Bentham Science Publishers; 2012. p. 3–16.
Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, et al. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology. 2017;77:261–74.
Van Der Vegt EJM, Van Der Ende J, Kirschbaum C, Verhulst FC, Tiemeier H. Early neglect and abuse predict diurnal cortisol patterns in adults. A study of international adoptees. Psychoneuroendocrinology. 2009;34:660–9.
Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry. 2009;66:69–75.
Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45.
Bunea IM, Szentágotai-Tǎtar A, Miu AC. Early-life adversity and cortisol response to social stress: a meta-analysis. Transl Psychiatry. 2017;7:1274.
Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–36.
Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the coronary artery risk development in young adults (CARDIA) Study. Psychosom Med. 2006;68:41–50.
Kumari M, Badrick E, Chandola T, Adler NE, Epel E, Seeman T, et al. Measures of social position and cortisol secretion in an aging population: findings from the Whitehall II study. Psychosom Med. 2010;72:27–34.
Karlamangla AS, Friedman EM, Seeman TE, Stawksi RS, Almeida DM. Daytime trajectories of cortisol: demographic and socioeconomic differences-Findings from the national study of daily experiences. Psychoneuroendocrinology. 2013;38:2585–97.
Dulin-Keita A, Casazza K, Fernandez JR, Goran MI, Gower B. Do neighbourhoods matter? Neighbourhood disorder and long-term trends in serum cortisol levels. J Epidemiol Community Health. 2012;66:24–29.
Wagner SL, Cepeda I, Krieger D, Maggi S, D’Angiulli A, Weinberg J, et al. Higher cortisol is associated with poorer executive functioning in preschool children: the role of parenting stress, parent coping and quality of daycare. Child Neuropsychol. 2016;22:853–69.
Chemin M, de Laat J, Haushofer J. Negative rainfall shocks increase levels of the stress hormone cortisol among poor farmers in Kenya. SSRN. 2013. https://doi.org/10.2139/ssrn.2294171.
Shapiro J, Haushofer J. The short-term impact of unconditional cash transfers to the poor: experimental evidence. Q J Econ. 2016;131:1973–2042.
Del Giudice M, Ellis B, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neurosci Biobehav Rev. 2011;35:1562–92.
Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary—developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301.
Bernard K, Frost A, Bennett CB, Lindhiem O. Maltreatment and diurnal cortisol regulation: a meta-analysis. Psychoneuroendocrinology. 2017;78:57–67.
Raffington L, Prindle J, Keresztes A, Binder J, Heim C, Shing YL. Blunted cortisol stress reactivity in low–income children relates to lower memory function. Psychoneuroendocrinology. 2018;90:110–21.
Hayward MD, Gorman BK. The long arm of childhod: the influence of early-life social conditions on men’s mortality. Demography. 2004;41:87–107.
Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–73.
Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81.
Clow A, Hucklebridge F, Stalder T, Clow P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev. 2010;35:97–103.
Elder GJ, Wetherell MA, Barclay NL, Ellis JG. The cortisol awakening response–applications and implications for sleep medicine. Sleep Med Rev. 2014;18:215–24.
Stavropoulos I, Pervanidou P, Gnardellis C, Loli N, Theodorou V, Mantzou A, et al. Epilepsy & behavior increased hair cortisol and antecedent somatic complaints in children with a fi rst epileptic seizure. Epilepsy Behav. 2017;68:146–52.
Noppe G, Van Rossum EFC, Vliegenthart J, Koper JW, Van Den Akker ELT. Elevated hair cortisol concentrations in children with adrenal insufficiency on hydrocortisone replacement therapy. Clin Endocrinol. 2014;81:820–5.
Föcker M, Stalder T, Kirschbaum C, Albrecht M, Adams F, De Zwaan M, et al. Hair cortisol concentrations in adolescent girls with anorexia nervosa are lower compared to healthy and psychiatric controls. Eur Eat Disord Rev. 2016;24:531–5.
Short SJ, Stalder T, Marceau K, Entringer S, Moog NK, Shirtcliff EA, et al. Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology. 2016;71:12–8.
Stalder T, Kirschbaum C. Analysis of cortisol in hair—State of the art and future directions. Brain Behav Immun. 2012;26:1019–29.
Tafet GE, Nemeroff CB. The links between stress and depression: psychoneuroendocrinological, genetic, and environmental interactions. J Neuropsychiatry Clin Neurosci. 2016;28:77–88.
Gray NA, Dhana A, Van Der Vyver L, Van Wyk J, Khumalo NP, Stein DJ. Determinants of hair cortisol concentration in children: a systematic review. Psychoneuroendocrinology. 2018;87:204–14.
Koss KJ, Gunnar MR. Annual research review: early adversity, the hypothalamic–pituitary–adrenocortical axis, and child psychopathology. J Child Psychol Psychiatry Allied Discip. 2018;59:327–46.
Lucas-Thompson RG, Henry KL, McKernan CJ. Is cortisol production in response to an acute stressor associated with diurnal cortisol production during adolescence? Dev Psychobiol. 2018. https://doi.org/10.1002/dev.21593.
Xie Q, Gao W, Li J, Qiao T, Jin J, Deng H, et al. Correlation of cortisol in 1-cm hair segment with salivary cortisol in human: hair cortisol as an endogenous biomarker. Clin Chem Lab Med. 2011;49:2013–9.
Vanaelst B, Huybrechts I, Bammann K, Michels N, de Vriendt T, Vyncke K, et al. Intercorrelations between serum, salivary, and hair cortisol and child-reported estimates of stress in elementary school girls. Psychophysiology. 2012;49:1072–81.
D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiol Behav. 2011;104:348–53.
Engelhardt LE, Church JA, Harden KP, Tucker-Drob EM. Accounting for the shared environment in cognitive abilities and academic achievement with measured socioecological contexts. Dev Sci. 2019;22:e12699.
Sonuga-Barke EJS, Kennedy M, Kumsta R, Knights N, Golm D, Rutter M, et al. Child-to-adult neurodevelopmental and mental health trajectories after early life deprivation: the young adult follow-up of the longitudinal English and Romanian Adoptees study. Lancet. 2017;389:1539–48.
Signorell A, Aho K, Alfons A, Anderegg N, Aragon T, Arppe A, et al. DescTools: tools for descriptive statistics. 2019.
OECD. In it together: why less inequality benefits all. Paris: OECD; 2015.
Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69:651–9.
Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–31.
Felt JM, Depaoli S, Tiemensma J. Latent growth curve models for biomarkers of the stress response. Front Neurosci. 2017;11:1–17.
Benjamini Y, Hochberg Y. Controlling the False discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300.
Grotzinger AD, Briley DA, Engelhardt LE, Mann FD, Patterson MW, Tackett JL, et al. Genetic and environmental influences on pubertal hormones in human hair across development. Psychoneuroendocrinology. 2018;90:76–84.
Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Dev Psychopathol. 2009;21:69–85.
Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–71.
Malanchini M, Engelhardt LE, Grotzinger AD, Harden KP, Tucker-Drob EM. “Same but different”: associations between multiple aspects of self-regulation, cognition and academic abilities. J Pers Soc Psychol. 2019;117:1164–88.
Hajat A, Moore K, Phuong Do D, Stein Merkin S, Punjabi NM, Sáñchez BN, et al. Examining the cross-sectional and longitudinal association between diurnal cortisol and neighborhood characteristics: evidence from the multi-ethnic study of atherosclerosis. Heal Place. 2015;34:199–206.
Theall KP, Shirtcliff EA, Dismukes AR, Wallace M, Dury SS. Association between neighborhood violence and biological stress in children. JAMA Pediatr. 2017;171:53–60.
Major JM, Doubeni CA, Freedman ND, Park Y, Lian M, Hollenbeck AR, et al. Neighborhood socioeconomic deprivation and mortality: NIH-AARP Diet and Health Study. PLoS ONE. 2010;5:e15538.
Hinson JP, Raven PW. Effects of endocrine-disrupting chemicals on adrenal function. Best Pract Res Clin Endocrinol Metab. 2006;20:111–20.
Shirtcliff EA, Peres JC, Dismukes AR, Lee Y, Phan JM. Riding the physiological roller coaster: adaptive significance of cortisol stress reactivity to social contexts. J Pers Disord. 2014;28:40–51.
Bouma EMC, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents’ cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. the trails study. Psychoneuroendocrinology. 2009;34:884–93.
Kidd T, Carvalho LA, Steptoe A. The relationship between cortisol responses to laboratory stress and cortisol profiles in daily life. Biol Psychol. 2014;99:34–40.
Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary- neurodevelopmental theory. Dev Psychopathol. 2011;23:7–28.
Shirtcliff EA, Skinner ML, Obasi EM, Haggerty KP. Positive parenting predicts cortisol functioning six years later in young adults. Dev Sci. 2017;20.
Freese J. The arrival of social science genomics. Contemp Sociol. 2018;47:524–36.
Dajani R, Hadfield K, van Uum S, Greff M, Panter-Brick C. Hair cortisol concentrations in war-affected adolescents: a prospective intervention trial. Psychoneuroendocrinology. 2018;89:138–46.
Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev Psychol. 2011;62:501–30.
Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, et al. Influence of early life stress on later hypothalamic—pituitary—adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Dev Psychopathol. 2011;23:1039–58.
Bor J, Cohen GH, Galea S. Population health in an era of rising income inequality: USA, 1980–2015. Lancet. 2017;389:1475–90.
Harden KP, Tucker-Drob EM, Tackett J. The Texas Twin Project. Twin Res Hum Genet. 2013;16:385–90.
Dettenborn L, Tietze A, Kirschbaum C, Stalder T. The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress. 2012;15:578–88.
Gao W, Stalder T, Foley P, Rauh M, Deng H, Kirschbaum C. Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. J Chromatogr B Anal Technol Biomed Life Sci. 2013;928:1–8.
Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, Kirschbaum C. Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology. 2012;37:602–10.
Grotzinger AD, Mann FD, Patterson MW, Tackett JL, Tucker-Drob EM, Harden KP. Hair and salivary testosterone, hair cortisol, and externalizing behaviors in adolescents. Psychol Sci. 2018. https://doi.org/10.1177/0956797617742981.
Porter B, Leary KDO. Marital discord and childhood behavioral problems. J Abnorm Child Psychol. 1980;8:287–95.
Muthén LK, Muthén BO. Mplus user’s guide. 8th ed. Los Angeles, CA: Muthén & Muthén; 2017.
Acknowledgements
We gratefully acknowledge all participant members of the Texas Twin Project. KPH and EMT-D are faculty research associates of the Population Research Center at the University of Texas at Austin, which is supported by a grant, 5-R24-HD042849, from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. KPH and EMT-D are also supported by Jacobs Foundation Research Fellowships. This research was supported by NIH grant R01HD083613. MM is partly supported by the David Wechsler Early Career Award for Innovative Work in Cognition and by NIH grant R01HD083613 awarded to EMT-D and KPH.
Author information
Authors and Affiliations
Contributions
MM, KPH, and EMT-D designed the study; MM, LEE, KPH, and EMT-D contributed new reagents/analytic tools; MM, LEE, and EMT-D analyzed data; MM, LEE, LR, AS, ADG, DAB, JWM, SMF, MWP, KPH, and EMT-D performed the research; and MM, KPH, and EMT-D wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Malanchini, M., Engelhardt, L.E., Raffington, L.A. et al. Weak and uneven associations of home, neighborhood, and school environments with stress hormone output across multiple timescales. Mol Psychiatry 26, 4823–4838 (2021). https://doi.org/10.1038/s41380-020-0747-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-0747-z