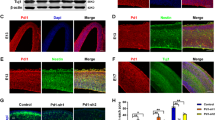
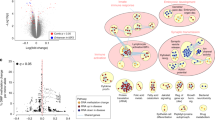
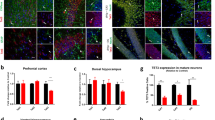
Abstract
Major depressive disorder (MDD) is the most common psychiatric disease worldwide. The precise molecular and cellular mechanisms underlying this disorder remain largely unknown. Wilms’ tumor 1 (Wt1), a transcription factor, plays critical roles in cancer and organ development. Importantly, deletion of the 11p13 region that contains the WT1 gene is a major cause of WARG syndrome (Wilms’ tumor, aniridia, genitourinary anomalies, and mental retardation), which is characterized by psychiatric disease, including depression. However, the roles and mechanisms of WT1 in embryonic neurogenesis and psychiatric disease remain unclear. Here, we demonstrate that the brain-specific deletion of Wt1 results in abnormal cell distribution during embryonic neurogenesis, which is accompanied by enhanced proliferation of neural progenitors and reduced neuronal differentiation. Moreover, neurons exhibit abnormal morphology during cortical development following Wt1 ablation. Furthermore, Wt1cKO mice exhibit depressive-like behaviors, including immobility, despair, and anhedonia. Mechanistically, Wt1 recruits Tet2 to the promoter of erythropoietin (Epo), which results in enhanced 5-hydroxymethylcytosine (5hmC) levels and the promotion of Epo expression. Either Epo plasmid electroporation or Epo protein injection can partially restore the deficiency caused by Wt1 deletion. Importantly, administration of Epo to both embryos and adults can ameliorate the depressive-like behavior of Wt1cKO mice. In addition, WT1 plays a similar role in human neural progenitor cells (hNPCs) proliferation and differentiation. Taken together, our findings reveal the critical role and regulatory mechanism of Wt1 in embryonic neurogenesis and behavioral modulation, which could contribute to the understanding of MDD etiology and therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Leng L, Zhuang K, Liu Z, Huang C, Gao Y, Chen G, et al. Menin deficiency leads to depressive-like behaviors in mice by modulating astrocyte-mediated neuroinflammation. Neuron. 2018;100:551–63. e557.
consortium C. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523:588–91.
Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547.
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–38.
Flint J, Kendler KS. The genetics of major depression. Neuron. 2014;81:484–503.
Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–62.
Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163:109–14.
Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15:80–92.
Li H, Zhu Y, Morozov YM, Chen X, Page SC, Rannals MD, et al. Disruption of TCF4 regulatory networks leads to abnormal cortical development and mental disabilities. Mol Psychiatry. 2019;24:1235–46.
Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66.
Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17.
Geerlings MI, Sigurdsson S, Eiriksdottir G, Garcia ME, Harris TB, Sigurdsson T, et al. Associations of current and remitted major depressive disorder with brain atrophy: the AGES-Reykjavik Study. Psychol Med. 2013;43:317–28.
Yang L, Han Y, Suarez Saiz F, Minden MD. A tumor suppressor and oncogene: the WT1 story. Leukemia. 2007;21:868–76.
Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, et al. WT-1 is required for early kidney development. Cell. 1993;74:679–91.
Bandiera R, Sacco S, Vidal VP, Chaboissier MC, Schedl A. Steroidogenic organ development and homeostasis: A WT1-centric view. Mol Cell Endocrinol. 2015;408:145–55.
Gao F, Maiti S, Alam N, Zhang Z, Deng JM, Behringer RR, et al. The Wilms tumor gene, Wt1, is required for Sox9 expression and maintenance of tubular architecture in the developing testis. Proc Natl Acad Sci USA. 2006;103:11987–92.
Martinez-Estrada OM, Lettice LA, Essafi A, Guadix JA, Slight J, Velecela V, et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet. 2010;42:89–93.
Wagner KD, Wagner N, Vidal VP, Schley G, Wilhelm D, Schedl A, et al. The Wilms’ tumor gene Wt1 is required for normal development of the retina. EMBO J. 2002;21:1398–405.
Wagner N, Wagner KD, Hammes A, Kirschner KM, Vidal VP, Schedl A, et al. A splice variant of the Wilms’ tumour suppressor Wt1 is required for normal development of the olfactory system. Development. 2005;132:1327–36.
Galvis-Blanco SJ, Arias-Florez JS, Contreras-Garcia GA. [WAGR syndrome by heterozygous deletion of the WT1 gene. Pediatric case report]. Arch Argent Pediatr. 2019;117:e505–8.
Zubenko GS, Maher B, Hughes HB 3rd, Zubenko WN, Stiffler JS, Kaplan BB, et al. Genome-wide linkage survey for genetic loci that influence the development of depressive disorders in families with recurrent, early-onset, major depression. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:1–18.
Shen T, Ji F, Yuan Z, Jiao J. CHD2 is required for embryonic neurogenesis in the developing cerebral cortex. Stem Cells. 2015;33:1794–806.
Zhang W, Wan H, Feng G, Qu J, Wang J, Jing Y, et al. SIRT6 deficiency results in developmental retardation in cynomolgus monkeys. Nature. 2018;560:661–5.
Weng YI, Huang TH, Yan PS. Methylated DNA immunoprecipitation and microarray-based analysis: detection of DNA methylation in breast cancer cell lines. Methods Mol Biol. 2009;590:165–76.
Cheng Y, Sun M, Chen L, Li Y, Lin L, Yao B, et al. Ten-eleven translocation proteins modulate the response to environmental stress in mice. Cell Rep. 2018;25:3194–203. e3194.
Ernst C. Proliferation and differentiation deficits are a major convergence point for neurodevelopmental disorders. Trends Neurosci. 2016;39:290–9.
Bernal A, Arranz L. Nestin-expressing progenitor cells: function, identity and therapeutic implications. Cell Mol Life Sci. 2018;75:2177–95.
Jelkmann W. Regulation of erythropoietin production. J Physiol. 2011;589:1251–8.
Beleslin-Cokic BB, Cokic VP, Yu X, Weksler BB, Schechter AN, Noguchi CT. Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood. 2004;104:2073–80.
Digicaylioglu M, Bichet S, Marti HH, Wenger RH, Rivas LA, Bauer C, et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci USA. 1995;92:3717–20.
Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007;64:159–71.
Kaneko N, Kako E, Sawamoto K. Enhancement of ventricular-subventricular zone-derived neurogenesis and oligodendrogenesis by erythropoietin and its derivatives. Front Cell Neurosci. 2013;7:235.
McPherson RJ, Juul SE. Erythropoietin for infants with hypoxic-ischemic encephalopathy. Curr Opin Pediatr. 2010;22:139–45.
Simon FH, Erhart P, Vcelar B, Scheuerle A, Schelzig H, Oberhuber A. Erythropoietin preconditioning improves clinical and histologic outcome in an acute spinal cord ischemia and reperfusion rabbit model. J Vasc Surg. 2016;64:1797–804.
Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–7.
Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–74.
Juul S. Erythropoietin in the central nervous system, and its use to prevent hypoxic-ischemic brain damage. Acta Paediatr Suppl. 2002;91:36–42.
Yu X, Shacka JJ, Eells JB, Suarez-Quian C, Przygodzki RM, Beleslin-Cokic B, et al. Erythropoietin receptor signalling is required for normal brain development. Development. 2002;129:505–16.
Hassouna I, Ott C, Wustefeld L, Offen N, Neher RA, Mitkovski M, et al. Revisiting adult neurogenesis and the role of erythropoietin for neuronal and oligodendroglial differentiation in the hippocampus. Mol Psychiatry. 2016;21:1752–67.
Dame C, Kirschner KM, Bartz KV, Wallach T, Hussels CS, Scholz H. Wilms tumor suppressor, Wt1, is a transcriptional activator of the erythropoietin gene. Blood. 2006;107:4282–90.
Wang Y, Xiao M, Chen X, Chen L, Xu Y, Lv L, et al. WT1 recruits TET2 to regulate its target gene expression and suppress leukemia cell proliferation. Mol Cell. 2015;57:662–73.
Chen F, Bertelsen AB, Holm IE, Nyengaard JR, Rosenberg R, Dorph-Petersen KA. Hippocampal volume and cell number in depression, schizophrenia, and suicide subjects. Brain Res. 2019;1727:146546.
Zuckerman H, Pan Z, Park C, Brietzke E, Musial N, Shariq AS, et al. Recognition and treatment of cognitive dysfunction in major depressive disorder. Front Psychiatry. 2018;9:655.
McIntyre RS, Xiao HX, Syeda K, Vinberg M, Carvalho AF, Mansur RB, et al. The prevalence, measurement, and treatment of the cognitive dimension/domain in major depressive disorder. CNS Drugs. 2015;29:577–89.
Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–31.
Li XB, Zheng W, Ning YP, Cai DB, Yang XH, Ungvari GS, et al. Erythropoietin for cognitive deficits associated with schizophrenia, bipolar disorder, and major depression: a systematic review. Pharmacopsychiatry. 2018;51:100–4.
Ma C, Cheng F, Wang X, Zhai C, Yue W, Lian Y, et al. Erythropoietin pathway: a potential target for the treatment of depression. Int J Mol Sci. 2016;17:677.
Osborn M, Rustom N, Clarke M, Litteljohn D, Rudyk C, Anisman H, et al. Antidepressant-like effects of erythropoietin: a focus on behavioural and hippocampal processes. PLoS ONE. 2013;8:e72813.
Acknowledgements
We thank all members in our lab for their valuable suggestions. We also thank Shiwen Li, Hua Qin, Xili Zhu, and Xuepei Lei for the help in confocal imaging and behavioral analysis. This work was supported by grants from the National Key R&D Program of China (2019YFA0110300), the CAS Strategic Priority Research Program (XDA16020602), the National Science Foundation of China (81825006, 31730033, and 31621004), Key deployment projects of the Chinese Academy of Sciences (ZDRW-ZS-2017-5), and K.C. Wong Education Foundation.
Author information
Authors and Affiliations
Contributions
FJ performed the experiments, analyzed the results, and wrote the manuscript. WW and CF provided the help in the experiment performance. FG provided the Wt1fl/fl mice and helped in Wt1 antibody test. JJ supervised the project and provided the funding support.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ji, F., Wang, W., Feng, C. et al. Brain-specific Wt1 deletion leads to depressive-like behaviors in mice via the recruitment of Tet2 to modulate Epo expression. Mol Psychiatry 26, 4221–4233 (2021). https://doi.org/10.1038/s41380-020-0759-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-0759-8
This article is cited by
-
TET2 deficiency promotes anxiety and depression-like behaviors by activating NLRP3/IL-1β pathway in microglia of allergic rhinitis mice
Molecular Medicine (2023)
-
Brain-specific Pd1 deficiency leads to cortical neurogenesis defects and depressive-like behaviors in mice
Cell Death & Differentiation (2023)
-
The role of neurotrophin genes involved in the vulnerability to gambling disorder
Scientific Reports (2022)