Abstract
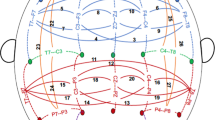
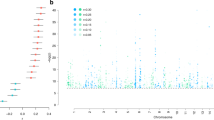
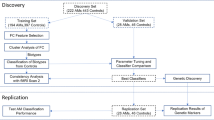
Aberrant connectivity of large-scale brain networks has been observed among individuals with alcohol use disorders (AUDs) as well as in those at risk, suggesting deficits in neural communication between brain regions in the liability to develop AUD. Electroencephalographical (EEG) coherence, which measures the degree of synchrony between brain regions, may be a useful measure of connectivity patterns in neural networks for studying the genetics of AUD. In 8810 individuals (6644 of European and 2166 of African ancestry) from the Collaborative Study on the Genetics of Alcoholism (COGA), we performed a Multi-Trait Analyses of genome-wide association studies (MTAG) on parietal resting-state theta (3–7 Hz) EEG coherence, which previously have been associated with AUD. We also examined developmental effects of GWAS findings on trajectories of neural connectivity in a longitudinal subsample of 2316 adolescent/young adult offspring from COGA families (ages 12–30) and examined the functional and clinical significance of GWAS variants. Six correlated single nucleotide polymorphisms located in a brain-expressed lincRNA (ENSG00000266213) on chromosome 18q23 were associated with posterior interhemispheric low theta EEG coherence (3–5 Hz). These same variants were also associated with alcohol use behavior and posterior corpus callosum volume, both in a subset of COGA and in the UK Biobank. Analyses in the subsample of COGA offspring indicated that the association of rs12954372 with low theta EEG coherence occurred only in females, most prominently between ages 25 and 30 (p < 2 × 10-9). Converging data provide support for the role of genetic variants on chromosome 18q23 in regulating neural connectivity and alcohol use behavior, potentially via dysregulated myelination. While findings were less robust, genome-wide associations were also observed with rs151174000 and parieto-frontal low theta coherence, rs14429078 and parieto-occipital interhemispheric high theta coherence, and rs116445911 with centro-parietal low theta coherence. These novel genetic findings highlight the utility of the endophenotype approach in enhancing our understanding of mechanisms underlying addiction susceptibility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Filbey FM. The American Journal of Drug and Alcohol Abuse An introduction to “The addiction connectome: brain connectivity in drug and alcohol addiction”. Am J Drug Alcohol Abus. 2013;39:341–2.
Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–68.
Nunez PL, Srinivasan R, Westdorp AF, Wijesinghe RS, Tucker DM, Silberstein RB, et al. EEG coherency: I: statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr Clin Neurophysiol. 1997;103:499–515.
Srinivasan R, Winter WR, Ding J, Nunez PL. EEG and MEG coherence: measures of functional connectivity at distinct spatial scales of neocortical dynamics. J Neurosci Methods. 2007;166:41–52.
Chorlian DB, Tang Y, Rangaswamy M, O’Connor S, Rohrbaugh J, Taylor R, et al. Heritability of EEG coherence in a large sib-pair population. Biol Psychol. 2007;75:260–6.
Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018.
Meyers JL, Zhang J, Wang JC, Su J, Kuo SI, Kapoor M, et al. An endophenotype approach to the genetics of alcohol dependence: a genome wide association study of fast beta EEG in families of African ancestry. Mol Psychiatry. 2017. https://doi.org/10.1038/mp.2016.239.
Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. Sci World J. 2007;7:131–41.
Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain Res. 2008;1235:153–71.
Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, et al. Variations in GABRA2, encoding the α2 subunit of the GABAA receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–14.
Malone SM, McGue M, Iacono WG. What can time-frequency and phase coherence measures tell us about the genetic basis of P3 amplitude? Int J Psychophysiol. 2017;115:40–56.
Iacono WG, Malone SM, Vaidyanathan U, Vrieze SI. Genome-wide scans of genetic variants for psychophysiological endophenotypes: a methodological overview. Psychophysiology. 2014;51:1207–24.
Vrieze SI, Malone SM, Pankratz N, Vaidyanathan U, Miller MB, Kang HM, et al. Genetic associations of nonsynonymous exonic variants with psychophysiological endophenotypes. Psychophysiology. 2014;51:1300–8.
Kang SJ, Rangaswamy M, Manz N, Wang J-C, Wetherill L, Hinrichs T, et al. Family-based genome-wide association study of frontal θ oscillations identifies potassium channel gene KCNJ6. Genes Brain Behav. 2012;11:712–9.
Smit DJA, Wright MJ, Meyers JL, Martin NG, Ho YYW, Malone SM, et al. Genome-wide association analysis links multiple psychiatric liability genes to oscillatory brain activity. Hum Brain Mapp. 2018;39:4183–95.
Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci USA. 2002;99:3729–33.
Whedon M, Perry NB, Calkins SD, Bell MA. Changes in frontal EEG coherence across infancy predict cognitive abilities at age 3: The mediating role of attentional control. Dev Psychol. 2016;52:1341–52.
Segalowitz SJ, Santesso DL, Jetha MK. Electrophysiological changes during adolescence: a review. Brain Cogn. 2010;72:86–100.
Chorlian DB, Rangaswamy M, Porjesz B. EEG coherence: topography and frequency structure. Exp Brain Res. 2009;198:59–83.
Duffy FH, Mcanulty GB, Albert MS. Effects of age upon interhemispheric EEG coherence in normal adults. Neurobiol Aging. 1996;17:587–99.
Rangaswamy M, Porjesz B. Understanding alcohol use disorders with neuroelectrophysiology. Handb Clin Neurol. 2014;125:383–414.
Cardenas VA, Price M, Fein G. EEG coherence related to fMRI resting state synchrony in long-term abstinent alcoholics. NeuroImage Clin. 2018;17:481–90.
Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naïve alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–64.
Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, et al. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15:418–27.
Hommer DW, Momenan R, Kaiser E, Rawlings RR. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158:198–204.
Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–89.
Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcohol Clin Exp Res. 1996;20:752–7.
Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage. 2002;15:708–18.
Pitel A-L, Chanraud S, Sullivan EV, Pfefferbaum A. Callosal microstructural abnormalities in Alzheimer’s disease and alcoholism: same phenotype, different mechanisms. Psychiatry Res Neuroimaging. 2010;184:49–56.
Clarke T-K, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N = 112 117). Mol Psychiatry. 2017;22:1376–84.
Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21:1656–69.
Chorlian DB, Rangaswamy M, Manz N, Meyers JL, Kang SJ, Kamarajan C, et al. Genetic correlates of the development of theta event related oscillations in adolescents and young adults. Int J Psychophysiol. 2017;115:24–39.
Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–58.
Travis F. Temporal and spatial characteristics of meditation EEG. Psychol Trauma Theory, Res Pract Policy. 2019. https://doi.org/10.1037/tra0000488.
Ahnaou A, Huysmans H, Jacobs T, Drinkenburg WHIM. Cortical EEG oscillations and network connectivity as efficacy indices for assessing drugs with cognition enhancing potential. Neuropharmacology. 2014;86:362–77.
Passynkova N, Neubauer H, Scheich H. Spatial organization of EEG coherence during listening to consonant and dissonant chords. Neurosci Lett. 2007;412:6–11.
Cook IA, O’Hara R, Uijtdehaage SH, Mandelkern M, Leuchter AF. Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalogr Clin Neurophysiol. 1998;107:408–14.
Wang J-C, Foroud T, Hinrichs AL, Le NXH, Bertelsen S, Budde JP, et al. A genome-wide association study of alcohol-dependence symptom counts in extended pedigrees identifies C15orf53. Mol Psychiatry. 2013;18:1218–24.
Baurley JW, Edlund CK, Pardamean CI, Conti DV, Bergen AW. Smokescreen: a targeted genotyping array for addiction research. BMC Genomics. 2016;17:145.
Delaneau O, Howie B, Cox AJ, Zagury J-F, Marchini J. Haplotype estimation using sequencing reads. Am J Hum Genet. 2013;93:687–96.
Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–7.
Wetherill L, Agrawal A, Kapoor M, Bertelsen S, Bierut LJ, Brooks A, et al. Association of substance dependence phenotypes in the COGA sample. Addict Biol. 2015;20:617–27.
Turley P, Walters RK, Maghzian O, Okbay A, Lee JJ, Fontana MA, et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat Genet. 2018;50:229–37.
Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–5.
Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1.
Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42:565–9.
de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol. 2015;11:e1004219.
Carithers LJ, Moore HM. The Genotype-Tissue Expression (GTEx) project. Biopreserv Biobank. 2015. https://doi.org/10.1038/ng.2653.
Martin JS, Xu Z, Reiner AP, Mohlke KL, Sullivan P, Ren B, et al. HUGIn: Hi-C unifying genomic interrogator. Bioinformatics. 2017;33:3793–5.
Schmitt AD, Hu M, Jung I, Xu Z, Qiu Y, Tan CL, et al. A compendium of chromatin contact maps reveals spatially active regions in the human genome. Cell Rep. 2016;17:2042–59.
Pandey AK, Ardekani BA, Kamarajan C, Zhang J, Chorlian DB, Byrne KN-H, et al. Lower prefrontal and hippocampal volume and diffusion tensor imaging differences reflect structural and functional abnormalities in abstinent individuals with alcohol use disorder. Alcohol Clin Exp Res. 2018. https://doi.org/10.1111/acer.13854. 17 August 2018.
Wang Z. Direct assessment of multiple testing correction in case-control association studies with related individuals. Genet Epidemiol. 2011;35:70–9.
Fanous AH, Zhou B, Aggen SH, Bergen SE, Amdur RL, Duan J, et al. Genome-wide association study of clinical dimensions of schizophrenia: polygenic effect on disorganized symptoms. Am J Psychiatry. 2012;169:1309–17.
Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA, et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature. 2016;533:539–42.
Vorstman JAS, Parr JR, Moreno-De-Luca D, Anney RJL, Nurnberger JI Jr, Hallmayer JF. Autism genetics: opportunities and challenges for clinical translation. Nat Rev Genet. 2017;18:362–76.
Farris SP, Arasappan D, Hunicke-Smith S, Harris RA, Mayfield RD. Transcriptome organization for chronic alcohol abuse in human brain. Mol Psychiatry. 2015;20:1438–47.
Zuo L, Tan Y, Wang Z, Wang K-S, Zhang X, Chen X, et al. Long noncoding RNAs in psychiatric disorders. Psychiatr Genet. 2016;26:109–16.
Sartor GC, St. Laurent G, Wahlestedt C. The emerging role of non-coding RNAs in drug addiction. Front Genet. 2012;3.
Spadaro PA, Flavell CR, Widagdo J, Ratnu VS, Troup M, Ragan C, et al. Long noncoding RNA-directed epigenetic regulation of gene expression is associated with anxiety-like behavior in mice. Biol Psychiatry. 2015;78:848–59.
Chen J, Bacanu S-A, Yu H, Zhao Z, Jia P, Kendler KS, et al. Genetic relationship between schizophrenia and nicotine dependence. Sci Rep. 2016;6:25671.
Warrington NM, Shevroja E, Hemani G, Hysi PG, Jiang Y, Auton A, et al. Genome-wide association study identifies nine novel loci for 2D:4D finger ratio, a putative retrospective biomarker of testosterone exposure in utero. Hum Mol Genet. 2018;27:2025–38.
Chen H, Sun J, Jiang H, Wang X, Wu L, Wu W, et al. Inferring alcoholism SNPs and regulatory chemical compounds based on ensemble bayesian network. Comb Chem High Throughput Screen. 2017;20:107–15.
Eeles R, Goh C, Castro E, Bancroft E, Guy M, Olama AAAl, et al. The genetic epidemiology of prostate cancer and its clinical implications. Nat Rev Urol. 2014;11:18–31.
Pottier C, Zhou X, Perkerson RB, Baker M, Jenkins GD, Serie DJ, et al. Potential genetic modifiers of disease risk and age at onset in patients with frontotemporal lobar degeneration and GRN mutations: a genome-wide association study. Lancet Neurol. 2018;17:548–58.
Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–86.
Kanai M, Akiyama M, Takahashi A, Matoba N, Momozawa Y, Ikeda M, et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat Genet. 2018;50:390–400.
Poggi G, Boretius S, Möbius W, Moschny N, Baudewig J, Ruhwedel T, et al. Cortical network dysfunction caused by a subtle defect of myelination. Glia. 2016;64:2025–40.
Li Z, Chen J, Yu H, He L, Xu Y, Zhang D, et al. Genome-wide association analysis identifies 30 new susceptibility loci for schizophrenia. Nat Genet. 2017;49:1576–83.
Kam JWY, Bolbecker AR, O’Donnell BF, Hetrick WP, Brenner CA. Resting state EEG power and coherence abnormalities in bipolar disorder and schizophrenia. J Psychiatr Res. 2013;47:1893–901.
Peterson RE, Kuchenbaecker K, Walters RK, Chen C-Y, Popejoy AB, Periyasamy S, et al. Genome-wide association studies in ancestrally diverse populations: opportunities, methods, pitfalls, and recommendations. Cell. 2019;179:589–603.
Khramtsova EA, Davis LK, Stranger BE. The role of sex in the genomics of human complex traits. Nat Rev Genet. 2019;20:173–90.
Meyers JL, Chorlian DB, Johnson EC, Pandey AK, Kamarajan C, Salvatore JE, et al. Association of polygenic liability for alcohol dependence and EEG connectivity in adolescence and young adulthood. Brain Sci. 2019;9:280.
Cousminer DL, Stergiakouli E, Berry DJ, Ang W, Groen-Blokhuis MM, Körner A, et al. Genome-wide association study of sexual maturation in males and females highlights a role for body mass and menarche loci in male puberty. Hum Mol Genet. 2014;23:4452–64.
CONVERGE consortium. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523:588–91.
Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Johnstone SJ, Hsu C-I, et al. Age and gender effects in EEG coherence: II. Boys with attention deficit/hyperactivity disorder. Clin Neurophysiol. 2005;116:977–84.
Barry RJ, Clarke AR, McCarthy R, Selikowitz M. Age and gender effects in EEG coherence: III. Girls with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2006;117:243–51.
Marosi E, Harmony T, Becker J, Bernal J, Reyes A, Rodriguez M, et al. Sex differences in EEG coherence in normal children. Int J Neurosci. 1993;72:115–21.
Costin BN, Miles MF. Molecular and neurologic responses to chronic alcohol use. Handb Clin Neurol. 2014;125:157–71.
Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol Psychiatry. 2006;59:364–72.
McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, et al. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33:1278–85.
Tozakidou M, Stippich C, Fischmann A. Teaching neuroimages: radiologic findings in Marchiafava-Bignami disease. Neurology. 2011;77:e67.
Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–15.
Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–22.
Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neurosci. 2004;10:372–92.
Alfonso-Loeches S, Pascual M, Guerri C. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology. 2013;311:27–34.
Welch KA, Carson A, Lawrie SM. Brain structure in adolescents and young adults with alcohol problems: systematic review of imaging studies. Alcohol Alcohol. 2013;48:433–44.
Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51:237–44.
Sokhadze TM, Cannon RL, Trudeau DL. EEG biofeedback as a treatment for substance use disorders: review, rating of efficacy, and recommendations for further research. Appl Psychophysiol Biofeedback. 2008;33:1–28.
Acknowledgements
This research is supported by U10AA008401 (BP), K01DA037914 (JLM), K01AA024152 (JES), K02DA32573 (AA), and K02AA018755 (DMD). The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B. Porjesz, V. Hesselbrock, T. Foroud; Scientific Director, A. Agrawal; Translational Director, D. Dick, includes eleven different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, T. Foroud, J. Nurnberger Jr., Y. Liu); University of Iowa (S. Kuperman, J. Kramer); SUNY Downstate (B. Porjesz, J. Meyers, C. Kamarajan, A. Pandey); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, A. Brooks, R. Hart); The Children’s Hospital of Philadelphia, University of Pennsylvania (L. Almasy); Virginia Commonwealth University (D. Dick, J. Salvatore); Icahn School of Medicine at Mount Sinai (A. Goate, M. Kapoor, P. Slesinger); and Howard University (D. Scott). Other COGA collaborators include: L. Bauer (University of Connecticut); L. Wetherill, X. Xuei, D. Lai, S. O’Connor, M. Plawecki, S. Lourens (Indiana University); L. Acion (University of Iowa); G. Chan (University of Iowa; University of Connecticut); D.B. Chorlian, J. Zhang, S. Kinreich, G. Pandey (SUNY Downstate); M. Chao (Icahn School of Medicine at Mount Sinai); A. Anokhin, V. McCutcheon, S. Saccone (Washington University); F. Aliev, P. Barr (Virginia Commonwealth University); H. Chin and A. Parsian are the NIAAA Staff Collaborators.We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting-Kai Li, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. We also thank Tim Bernard Bigdeli for his assistance with this manuscript. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Unrelated to this work, coauthor AA received peer-reviewed funding, travel and an honorarium from ABMRF (end 12/2012) which receives support from the brewing industry. We have no other conflicts of interest to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Meyers, J.L., Zhang, J., Chorlian, D.B. et al. A genome-wide association study of interhemispheric theta EEG coherence: implications for neural connectivity and alcohol use behavior. Mol Psychiatry 26, 5040–5052 (2021). https://doi.org/10.1038/s41380-020-0777-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-0777-6
This article is cited by
-
Electroencephalogram Coherence and Peripheral Markers of Nervous Tissue Damage in Depressive Disorders
Neuroscience and Behavioral Physiology (2023)
-
The correlation between upper body grip strength and resting-state EEG network
Medical & Biological Engineering & Computing (2023)