Abstract
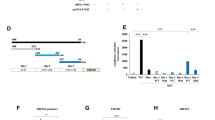
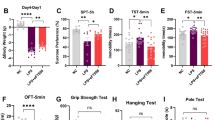
Major depression disorder is a severe mental illness often linked with metabolic disorders. Adiponectin is an adipocyte-secreted circulatory hormone with antidiabetic and glucose/lipid modulation capacities. Studies have demonstrated the pathophysiological roles of adiponectin involved in various neurological disorders, including depression. However, the underlying mechanisms are poorly understood. Here we showed that adiponectin deprivation enhanced antidepressive-like behaviors in the LPS-induced model of depression. APN KO mice displayed increased cytokines (both pro and anti-inflammatory), accompanied by an impaired expression of adiponectin receptors (mRNA/protein level) and decreasing IBA-1 level in the cortex and primary microglia of LPS treated APN KO mice. Further, LPS-treatment significantly reduced p-NFκB expression in the microglia of APN KO mice. However, the Bay11-7082 treatment recovered p-NFκB expression in the cortex of APN KO mice in the presence of LPS. Interestingly, the antidepressant potentials of APN KO mice were abolished by TrkB antagonist K252a, IKK inhibitor Bay11-7082, and AdipoRon suggesting crosstalk between TrkB/BDNF signaling and NFκB in depression. Furthermore, the effects of Bay11-7082 were abolished by a TrkB/BDNF activator (7,8-DHF), indicating a critical role of TrkB/BDNF signaling. Taken together, these findings showed that dysregulated neuroinflammatory status and BDNF signaling might underlie the antidepressive-like behaviors of APN KO mice. NFκB elicited BDNF changes may be accountable for the pathogenesis of LPS induced depression, where APN might present an alternative therapeutic target for depressive disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files.
References
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25.
Sjöberg L, Karlsson B, Atti AR, Skoog I, Fratiglioni L, Wang HX. Prevalence of depression: comparisons of different depression definitions in population-based samples of older adults. J Affect Disord. 2017;221:123–31.
Dean J, Keshavan M. The neurobiology of depression: an integrated view. Asian J psychiatry. 2017;27:101–11.
Blier P. Neurobiology of depression and mechanism of action of depression treatments. J Clin psychiatry. 2016;77:e319.
Dowlati Y, Herrmann N, Swardfager WL, Reim EK, Lanctôt KL. Efficacy and tolerability of antidepressants for treatment of depression in coronary artery disease: a meta-analysis. Can J psychiatry Rev canadienne de Psychiatr. 2010;55:91–99.
Brent DA. Antidepressants and suicidality. Psychiatr Clin North Am. 2016;39:503–12.
Agius M, Bonnici H. Antidepressants in use in clinical practice. Psychiatr Danubina. 2017;29(Suppl 3):667–71.
Ghoneim MM, O’Hara MW. Depression and postoperative complications: an overview. BMC Surg. 2016;16:5.
Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902.
Villas Boas GR, Boerngen de Lacerda R, Paes MM, Gubert P, Almeida WLDC, Rescia VC, et al. Molecular aspects of depression: a review from neurobiology to treatment. Eur J Pharmacol. 2019;851:99–121.
Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17:497–511.
Dantzer R. Depression and inflammation: an intricate relationship. Biol psychiatry. 2012;71:4–5.
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol psychiatry. 2010;67:446–57.
Sestan-Pesa M, Horvath TL. Metabolism and mental illness. Trends Mol Med. 2016;22:174–83.
Malynn S, Campos-Torres A, Moynagh P, Haase J. The pro-inflammatory cytokine TNF-α regulates the activity and expression of the serotonin transporter (SERT) in astrocytes. Neurochem Res. 2013;38:694–704.
Kabiersch A, del Rey A, Honegger CG, Besedovsky HO. Interleukin-1 induces changes in norepinephrine metabolism in the rat brain. Brain, Behav, Immun. 1988;2:267–74.
Jeon SW, Kim YK. Neuroinflammation and cytokine abnormality in major depression: cause or consequence in that illness? World J Psychiatry. 2016;6:283–93.
Rubartelli A. Redox control of NLRP3 inflammasome activation in health and disease. J Leukoc Biol. 2012;92:951–8.
Dai Y, Zhang J, Xiang J, Li Y, Wu D, Xu J. Calcitriol inhibits ROS-NLRP3-IL-1β signaling axis via activation of Nrf2-antioxidant signaling in hyperosmotic stress stimulated human corneal epithelial cells. Redox Biol. 2019;21:101093.
Zhang B, Zhao J, Wang Z, Xu L, Liu A, Du G. DL0410 attenuates oxidative stress and neuroinflammation via BDNF/TrkB/ERK/CREB and Nrf2/HO-1 activation. Int Immunopharmacol. 2020;86:106729.
van Leeuwen E, Hampton MB, Smyth LCD. Redox signalling and regulation of the blood-brain barrier. Int J Biochem Cell Biol. 2020;125:105794.
Aguilera G, Colín-González AL, Rangel-López E, Chavarría A, Santamaría A. Redox signaling, neuroinflammation, and neurodegeneration. Antioxid redox Signal. 2018;28:1626–51.
Resende R, Fernandes T, Pereira AC, De Pascale J, Marques AP, Oliveira P, et al. Mitochondria, endoplasmic reticulum and innate immune dysfunction in mood disorders: do mitochondria-associated membranes (MAMs) play a role? Biochimica et Biophysica Acta Mol basis Dis. 2020;1866:165752.
Torres-Odio S, Key J, Hoepken HH, Canet-Pons J, Valek L, Roller B, et al. Progression of pathology in PINK1-deficient mouse brain from splicing via ubiquitination, ER stress, and mitophagy changes to neuroinflammation. J neuroinflammation. 2017;14:154.
Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–17.
Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen psychiatry. 2010;67:360–8.
Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–95.
Kaufmann FN, Costa AP, Ghisleni G, Diaz AP, Rodrigues ALS, Peluffo H, et al. NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav Immun. 2017;64:367–83.
Pal China S, Sanyal S, Chattopadhyay N. Adiponectin signaling and its role in bone metabolism. Cytokine. 2018;112:116–31.
Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–26.
Straub LG, Scherer PE. Metabolic messengers: adiponectin. Nat Metab. 2019;1:334–9.
Rizzo MR, Fasano R, Paolisso G. Adiponectin and Cognitive Decline. Int j mol sci. 2020; 21:2010.
Liu J, Guo M, Zhang D, Cheng S-Y, Liu M, Ding J, et al. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc Natl Acad Sci. 2012;109:12248–53.
Hu Y, Dong X, Chen J. Adiponectin and depression: a meta-analysis. Biomed Res. 2015;3:38–42.
Li C, Meng F, Garza JC, Liu J, Lei Y, Kirov SA et al. Modulation of depression-related behaviors by adiponectin AdipoR1 receptors in 5-HT neurons. Mol psychiatry 2020. https://www.nature.com/articles/s41380-020-0649-0
Rastegar S, Parimisetty A, Cassam Sulliman N, Narra SS, Weber S, Rastegar M, et al. Expression of adiponectin receptors in the brain of adult zebrafish and mouse: Links with neurogenic niches and brain repair. J Comp Neurol. 2019;527:2317–33.
Lee TH, Cheng KK, Hoo RL, Siu PM, Yau SY. The novel perspectives of adipokines on brain health. Int J mol sci 2019; 20:5638.
Bloemer J, Pinky PD, Smith WD, Bhattacharya D, Chauhan A, Govindarajulu M et al. Adiponectin knockout mice display cognitive and synaptic deficits. Front Endocrinol 2019;10:819.
Li W, Ali T, He K, Liu Z, Shah FA, Ren Q et al. Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain Behav Immun. 2020;10:819.
Ali T, Rahman SU, Hao Q, Li W, Liu Z, Ali Shah F et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J pineal res. 2020;69:e12667.
Ali T, Rahman SU, Hao Q, Li W, Liu Z, Ali Shah F, et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J Pineal Res. 2020;69:e12667.
Zhao X, Cao F, Liu Q, Li X, Xu G, Liu G, et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behavioural brain Res. 2019;364:494–502.
Wang M, Ye X, Hu J, Zhao Q, Lv B, Ma W, et al. NOD1/RIP2 signalling enhances the microglia-driven inflammatory response and undergoes crosstalk with inflammatory cytokines to exacerbate brain damage following intracerebral haemorrhage in mice. J Neuroinflammation. 2020;17:364.
Dai X, Sun Y, Jiang Z. Protective effects of vitamin E against oxidative damage induced by Abeta1-40Cu(II) complexes. Acta Biochimica et Biophysica Sin. 2007;39:123–30.
Li YH, Yan ZQ, Jensen JS, Tullus K, Brauner A. Activation of nuclear factor kappaB and induction of inducible nitric oxide synthase by Ureaplasma urealyticum in macrophages. Infect Immun. 2000;68:7087–93.
Shah SA, Khan M, Jo MH, Jo MG, Amin FU, Kim MO. Melatonin stimulates the SIRT 1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)‐induced oxidative stress to rescue postnatal rat brain. CNS Neurosci therapeutics. 2017;23:33–44.
Arifin WN, Zahiruddin WM. Sample size calculation in animal studies using resource equation approach. Malays J Med Sci. 2017;24:101–5.
Mead R, Gilmour SG, Mead A Statistical Principles for the Design of Experiments: Applications to Real Experiments. Cambridge University Press: Cambridge, 2012.
Li W, Ali T, He K, Liu Z, Shah FA, Ren Q, et al. Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain, Behav, Immun. 2021;92:10–24.
Singla B, Holmdahl R, Csanyi G. Editorial: oxidants and redox signaling in inflammation. Front Immunol. 2019;10:545.
Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–59.
Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–9.
Holmes SE, Scheinost D, Finnema SJ, Naganawa M, Davis MT, DellaGioia N, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun. 2019;10:1529.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49.
Li W, Ali T, Zheng C, Liu Z, He K, Shah FA, et al. Fluoxetine regulates eEF2 activity (phosphorylation) via HDAC1 inhibitory mechanism in an LPS-induced mouse model of depression. J Neuroinflammation. 2021;18:38.
Caviedes A, Lafourcade C, Soto C, Wyneken U. BDNF/NF-κB signaling in the neurobiology of depression. Curr Pharm Des. 2017;23:3154–63.
Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012;165:313–27.
Jo D, Son Y, Yoon G, Song J, Kim OY. Role of adiponectin and brain derived neurotrophic factor in metabolic regulation involved in adiposity and body fat browning. J Clin Med. 2020;10:56.
Shelton RC, Falola M, Li L, Zajecka J, Fava M, Papakostas GI. The pro-inflammatory profile of depressed patients is (partly) related to obesity. J Psychiatr Res. 2015;70:91–97.
Jeong HG, Min BJ, Lim S, Kim TH, Lee JJ, Park JH, et al. Plasma adiponectin elevation in elderly individuals with subsyndromal depression. Psychoneuroendocrinology. 2012;37:948–55.
Diniz BS, Teixeira AL, Campos AC, Miranda AS, Rocha NP, Talib LL, et al. Reduced serum levels of adiponectin in elderly patients with major depression. J Psychiatr Res. 2012;46:1081–5.
Liu J, Guo M, Zhang D, Cheng SY, Liu M, Ding J, et al. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc Natl Acad Sci USA. 2012;109:12248–53.
Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86.
Lamers F, Milaneschi Y, de Jonge P, Giltay EJ, Penninx B. Metabolic and inflammatory markers: associations with individual depressive symptoms. Psychological Med. 2018;48:1102–10.
Black C, Miller BJ. Meta-analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol psychiatry. 2015;78:28–37.
Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol: J Eur Coll Neuropsychopharmacol. 2015;25:1532–43.
Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scandinavica. 2017;135:373–87.
Walker AK, Wing EE, Banks WA, Dantzer R. Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol psychiatry. 2019;24:1523–32.
Tomaz VS, Chaves Filho AJM, Cordeiro RC, Jucá PM, Soares MVR, Barroso PN, et al. Antidepressants of different classes cause distinct behavioral and brain pro- and anti-inflammatory changes in mice submitted to an inflammatory model of depression. J Affect Disord. 2020;268:188–200.
Guo LT, Wang SQ, Su J, Xu LX, Ji ZY, Zhang RY, et al. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J Neuroinflammation. 2019;16:95.
Sakai J, Cammarota E, Wright JA, Cicuta P, Gottschalk RA, Li N, et al. Lipopolysaccharide-induced NF-κB nuclear translocation is primarily dependent on MyD88, but TNFα expression requires TRIF and MyD88. Sci Rep. 2017;7:1428.
Weisz F, Piccinin S, Mango D, Ngomba RT, Mercuri NB, Nicoletti F et al. The role of adiponectin receptors in the regulation of synaptic transmission in the hippocampus. Synapse 2017;71:21964.
Zheng J, Sun Z, Liang F, Xu W, Lu J, Shi L, et al. AdipoRon attenuates neuroinflammation after intracerebral hemorrhage through AdipoR1-AMPK pathway. Neuroscience. 2019;412:116–30.
Koh EH, Park JY, Park HS, Jeon MJ, Ryu JW, Kim M, et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007;56:2973–81.
Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Pr Res Clin Endocrinol Metab. 2014;28:15–23.
Nicolas S, Debayle D, Béchade C, Maroteaux L, Gay AS, Bayer P, et al. Adiporon, an adiponectin receptor agonist acts as an antidepressant and metabolic regulator in a mouse model of depression. Transl Psychiatry. 2018;8:159.
Acknowledgements
Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions, Shenzhen, 518055, China. This work was supported by Grants Science and Technology Innovation Committee of Shenzhen No: JCYJ20170810163329510; Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions No: 2019SHIBS0004; Sanming Project of Medicine in Shenzhen (No. SZSM201911003) Shenzhen Key Medical Discipline Construction Fund (No.SZXK06162); Shenzhen bay laboratory NO:SZBL2019062801003.
Author information
Authors and Affiliations
Contributions
WL designed and performed the experiments, TA data analysis, wrote the manuscript, Chengyou Zheng, Kaiwu He, and Zizhen Liu helped in the experiment; Fawad Ali Shah, Ningning Li, and Zhi-Jian Yu helped in manuscript, experimental tools and supported the study. SL endorsed the study, corresponding authors, reviewed and approved the manuscript, and held all the responsibilities related to this manuscript. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
According to the protocols approved by the Institutional Animal Care and Use Committee of Peking University Shenzhen Graduate School, all experimental procedures were carried out.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, W., Ali, T., Zheng, C. et al. Anti-depressive-like behaviors of APN KO mice involve Trkb/BDNF signaling related neuroinflammatory changes. Mol Psychiatry 27, 1047–1058 (2022). https://doi.org/10.1038/s41380-021-01327-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01327-3
This article is cited by
-
Exploring the mechanism of Icariin in the treatment of depression through BDNF-TrkB pathway based on network pharmacology
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
The Interaction Between Nutraceuticals and Gut Microbiota: a Novel Therapeutic Approach to Prevent and Treatment Parkinson’s Disease
Molecular Neurobiology (2024)
-
Enhanced antidepressant effects of BDNF-quercetin alginate nanogels for depression therapy
Journal of Nanobiotechnology (2023)
-
Adiponectin deficiency accelerates brain aging via mitochondria-associated neuroinflammation
Immunity & Ageing (2023)
-
Human umbilical cord-derived mesenchymal stem cells ameliorate perioperative neurocognitive disorder by inhibiting inflammatory responses and activating BDNF/TrkB/CREB signaling pathway in aged mice
Stem Cell Research & Therapy (2023)