Abstract
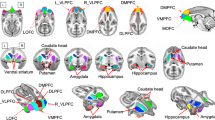
Stress affects brain serotonin (5HT) and dopamine (DA) function, and the effectiveness of 5HT and DA to regulate stress and emotional responses. However, our understanding of the long-term impact of early life adversity (ELA) on primate brain monoaminergic systems during adolescence is scarce and inconsistent. Filling this gap in the literature is critical, given that the emergence of psychopathology during adolescence has been related to deficits in these systems. Here, we use a translational nonhuman primate (NHP) model of ELA (infant maltreatment by the mother) to examine the long-term impact of ELA on adolescent 5HT1A, 5HT2A and D2 receptor systems. These receptor systems were chosen based on their involvement in stress/emotional control, as well as reward and reinforcement. Rates of maternal abuse, rejection, and infant’s vocalizations were obtained during the first three postnatal months, and hair cortisol concentrations obtained at 6 months postnatal were examined as early predictors of binding potential (BP) values obtained during adolescence using positron emission tomography (PET) imaging. Maltreated animals demonstrated significantly lower 5HT1A receptor BP in prefrontal cortical areas as well as the amygdala and hippocampus, and lower 5HT2A receptor BP in striatal and prefrontal cortical areas. Maltreated animals also demonstrated significantly lower D2 BP in the amygdala. None of the behavioral and neuroendocrine measurements obtained early in life predicted any changes in BP data. Our findings suggest that early caregiving experiences regulate the development of brain 5HT and DA systems in primates, resulting in long-term effects evident during adolescence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82:217–25.
Carr CP, Martins CMS, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis. 2013;201:1007–20.
Cicchetti D, Toth SL. Child maltreatment. Annu Rev Clin Psychol. 2005;1:409–38.
Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Mol Psychiatry. 2014;19:544–54.
Drury SS, Sanchez MM, Gonzalez A. When mothering goes awry: challenges and opportunities for utilizing evidence across rodent, nonhuman primate and human studies to better define the biological consequences of negative early caregiving. Horm Behav. 2016;77:182–92.
Finkelhor D, Turner HA, Shattuck A, Hamby SL. Violence, crime, and abuse exposure in a national sample of children and youth: an update. JAMA Pediatr. 2013;167:614–21.
Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp Neurol. 2012;233:102–11.
Kaplow JB, Widom CS. Age of onset of child maltreatment predicts long-term mental health outcomes. J Abnorm Psychol. 2007;116:176–87.
Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44.
Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115:424–43.
Forbes EE, Williamson DE, Ryan ND, Dahl RE. Positive and negative affect in depression: influence of sex and puberty. Ann NY Acad Sci. 2004;1021:341–47.
Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57.
Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–35.
Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–37.
Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003;37:357–73.
Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, et al. In vivo PET study of 5HT2A serotonin and D2 dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42:306–14.
Denys D, Van Der Wee N, Janssen J, De Geus F, Westenberg HG. Low level of dopaminergic D2 receptor binding in obsessive-compulsive disorder. Biol Psychiatry. 2004;55:1041–5.
Schneier FR, Abi‐Dargham A, Martinez D, Slifstein M, Hwang DR, Liebowitz MR, et al. Dopamine transporters, D2 receptors, and dopamine release in generalized social anxiety disorder. Depress Anxiety. 2009;26:411–8.
Moses-Kolko EL, Price JC, Wisner KL, Hanusa BH, Meltzer CC, Berga SL, et al. Postpartum and depression status are associated with lower [(11)C]raclopride BP(ND) in reproductive-age women. Neuropsychopharmacology. 2012;37:1422–32.
Drury SS, Howell BR, Jones C, Esteves K, Morin E, Schlesinger R, et al. Shaping long-term primate development: telomere length trajectory as an indicator of early maternal maltreatment and predictor of future physiologic regulation. Dev Psychopathol. 2017;29:1539–51.
Howell BR, McCormack KM, Grand AP, Sawyer NT, Zhang X, Maestripieri D, et al. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: associations with high cortisol during infancy. Biol Mood Anxiety Disord. 2013;3:21.
Howell BR, Grand AP, McCormack KM, Shi Y, La Prairie J, Maestripieri D, et al. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: relation to Amygdala volume. Dev Psychobiol. 2014;56:1735–46.
McCormack K, Sanchez MM, Bardi M, Maestripieri D. Maternal care patterns and behavioral development of rhesus macaque abused infants in the first 6 months of life. Dev Psychobiol. 2006;48:537–50.
Morin EL, Howell BR, Meyer JS, Sanchez MM. Effects of early maternal care on adolescent attention bias to threat in nonhuman primates. Dev Cogn Neurosci. 2019;38:100643.
Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack KM, Sanchez MM. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques (Macaca mulatta). Behav Neurosci 2006a;120:1017.
Maestripieri D, McCormack K, Lindell SG, Higley JD, Sanchez MM. Influence of parenting style on the offspring’s behaviour and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behav Brain Res. 2006b;175:90–5.
Sanchez MM, Alagbe O, Felger JC, Zhang J, Graff AE, Grand AP, et al. Activated p38 MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Mol Psychiatry. 2007;12:895–7.
Huggins KN, Mathews TA, Locke JL, Szeliga KT, Friedman DP, Bennett AJ, et al. Effects of early life stress on drinking and serotonin system activity in rhesus macaques: 5-Hydroxyindoleacetic acid in cerebrospinal fluid predicts brain tissue levels. Alcohol. 2012;46:371–6.
Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–74.
Nader MA, Czoty PW. PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry. 2005;162:1473–82.
Embree M, Michopoulos V, Votaw JR, Voll RJ, Mun J, Stehouwer JS, et al. The relation of developmental changes in brain serotonin transporter (5HTT) and 5HT1A receptor binding to emotional behavior in female rhesus monkeys: effects of social status and 5HTT genotype. Neuroscience. 2013;228:83–100.
Spinelli S, Chefer S, Carson RE, Jagoda E, Lang L, Heilig M, et al. Effects of early-life stress on serotonin1A receptors in juvenile rhesus monkeys measured by positron emission tomography. Biol Psychiatry. 2010;67:1146–53.
Howell BR, McMurray MS, Guzman DB, Nair G, Shi Y, McCormack KM, et al. Maternal buffering beyond glucocorticoids: impact of early life stress on corticolimbic structures that control infant responses to novelty. Soc Neurosci. 2017;12:50–64.
Howell BR, Ahn M, Shi Y, Godfrey JR, Hu X, Zhu H, et al. Disentangling the effects of early caregiving experience and heritable factors on brain white matter development in rhesus monkeys. NeuroImage. 2019;197:625–42.
Morin EL, Howell BR, Feczko E, Earl E, Pincus M, Reding K, et al. Developmental outcomes of early adverse care on amygdala functional connectivity in nonhuman primates. Dev Psychopathol. 2020;32:1579–96.
Wakeford AGP, Morin EL, Bramlett SN, Howell BR, McCormack KM, Meyer JS, et al. Effects of early life stress on cocaine self-administration in post-pubertal male and female rhesus macaques. Psychopharmacology. 2019;236:2785–96.
Müller CP, Carey RJ, Huston JP, Silva MADS. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog Neurobiol. 2007;81:133–78.
Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67:176–97.
Maestripieri D, Megna NL, Jovanovic T. Adoption and maltreatment of foster infants by rhesus macaque abusive mothers. Dev Sci. 2000;3:287–93.
Maestripieri D. Parenting styles of abusive mothers in group-living rhesus macaques. Anim Behav. 1998;55:1–11.
McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm Behav. 2009;55:538–47.
McCormack KM, Howell BR, Higgins M, Bramlett S, Guzman D, Morin EL, et al. The developmental consequences of early adverse care on infant macaques: a cross-fostering study. Psychoneuroendocrinology. 2022;146:105947 https://doi.org/10.1016/j.psyneuen.2022.105947.
McCormack K, Howell BR, Guzman D, Villongco C, Pears K, Kim H, et al. The development of an instrument to measure global dimensions of maternal care in rhesus macaques (Macaca mulatta). Am J Primatol. 2015;77:20–33.
Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav. 2006;50:623–31.
Maestripieri D. The biology of human parenting: insights from nonhuman primates. Neurosci Biobehav Rev. 1999;23:411–22.
Scheinin M. Monoamine metabolites in human cerebrospinal fluid: indicators of neuronal activity? Med Biol. 1985;63:1–17.
Sodhi MSK, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–74.
Mugler JP 3rd. Overview of MR imaging pulse sequences. Magn Reson Imaging Clin N. Am. 1999;7:661.
Lüsebrink F, Wollrab A, Speck O. Cortical thickness determination of the human brain using high resolution 3T and 7T MRI data. Neuroimage. 2013;70:122–31.
Mukherjee J, Yang ZY, Brown T, Lew R, Wernick M, Ouyang X, et al. Preliminary assessment of extrastriatal dopamine D-2 receptor binding in the rodent and nonhuman primate brains using the high affinity radioligand, 18F-fallypride. Nucl Med Biol. 1999;26:519–27.
Le Bars D, Lemaire C, Ginovart N, Plenevaux A, Aerts J, Brihaye C, et al. High-yield radiosynthesis and preliminary in vivo evaluation of p-[18F]MPPF, a fluoro analog of WAY-100635. Nucl Med Biol. 1998;25:343–50.
Ito H, Nyberg S, Halldin C, Lundkvist C, Farde L. PET imaging of central 5-HT2A receptors with carbon-11-MDL 100,907. J Nucl Med. 1998;39:208–14.
Yang Y, Tai YC, Siegel S, Newport DF, Bai B, Li Q, et al. Optimization and performance evaluation of the microPET II scanner for in vivo small-animal imaging. Phys Med Biol. 2004;49:2527–45.
Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–33.
Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–98.
Shi Y, Budin F, Yapuncich E, Rumple A, Young JT, Payne C, et al. UNC-Emory infant atlases for macaque brain image analysis: postnatal brain development through 12 months. Front Neurosci. 2017;10:617.
McLaren DG, Kosmatka KJ, Kastman EK, Bendlin BB, Johnson SC. Rhesus macaque brain morphometry: a methodological comparison of voxel-wise approaches. Methods. 2010;50:157–65.
Michopoulos V, Embree M, Reding K, Sanchez MM, Toufexis D, Votaw JR, et al. CRH receptor antagonism reverses the effect of social subordination upon central GABAA receptor binding in estradiol-treated ovariectomized female rhesus monkeys. Neuroscience. 2013;250:300–8.
Michopoulos V, Perez Diaz M, Embree M, Reding K, Votaw JR, Mun J, et al. Oestradiol alters central 5‐HT 1A receptor binding potential differences related to psychosocial stress but not differences related to 5‐HTTLPR genotype in female rhesus monkeys. J Neuroendocrinol. 2014;26:80–8.
Christian BT, Vandehey NT, Fox AS, Murali D, Oakes TR, Converse AK, et al. The distribution of D2/D3 receptor binding in the adolescent rhesus monkey using small animal PET imaging. Neuroimage. 2009;44:1334–44.
Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, et al. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:42–52.
Smith CT, Crawford JL, Dang LC, Seaman KL, San Juan MD, Vijay A, et al. Partial-volume correction increases estimated dopamine D2-like receptor binding potential and reduces adult age differences. J Cereb Blood Flow Metab. 2019;39:822–33.
Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–77.
Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, et al. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–91.
Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, et al. Serotonin 5-HT 1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193:229–34.
Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psych. 2007;61:1081–9.
Adams KH, Hansen ES, Pinborg LH, Hasselbalch SG, Svarer C, Holm S, et al. Patients with obsessive–compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int J Neuropsychopharmacol. 2005;8:391–401.
Soloff PH, Price JC, Meltzer CC, Fabio A, Frank GK, Kaye WH. 5HT2A receptor binding is increased in borderline personality disorder. Biol Psych. 2007;62:580–7.
Mintun MA, Sheline YI, Moerlein SM, Vlassenko AG, Huang Y, Snyder AZ. Decreased hippocampal 5-HT2A receptor binding in major depressive disorder: in vivo measurement with [18F] altanserin positron emission tomography. Biol Psych. 2004;55:217–24.
Godfrey JR, Diaz MP, Pincus M, Kovacs-Balint Z, Feczko E, Earl E, et al. Diet matters: glucocorticoid-related neuroadaptations associated with calorie intake in female rhesus monkeys. Psychoneuroendocrinology. 2018;91:169–78.
Michopoulos V, Perez Diaz M, Wilson ME. Social change and access to a palatable diet produces differences in reward neurochemistry and appetite in female monkeys. Physiol Biol. 2016;162:102–11.
Leventopoulos M, Russig H, Feldon J, Pryce CR, Opacka-Juffry J. Early deprivation leads to long-term reductions in motivation for reward and 5-HT1A binding and both effects are reversed by fluoxetine. Neuropharmacology 2009;56:692–701.
Rentesi G, Antoniou K, Marselos M, Syrrou M, Papadopoulou-Daifoti Z, Konstandi M. Early maternal deprivation-induced modifications in the neurobiological, neurochemical and behavioral profile of adult rats. Behav Brain Res. 2013;244:29–37.
Lee SH, Jung EM. Adverse effects of early-life stress: focus on the rodent neuroendocrine system. Neural Regen Res. 2024;19:336–41.
Bravo JA, Dinan TG, Cryan JF. Early-life stress induces persistent alterations in 5-HT1A receptor and serotonin transporter mRNA expression in the adult rat brain. Front Mol Neurosci. 2014;7:24.
Vazquez DM, Lopez JF, Van Hoers H, Watson SJ, Levine S. Maternal deprivation regulates serotonin 1A and 2A receptors in the infant rat. Brain Res. 2000;855:76–82.
Berton O, Aguerre S, Sarrieau A, Mormede P, Chaouloff F. Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience. 1998;82:147–59.
Dwivedi Y, Mondal AC, Payappagoudar GV, Rizavi HS. Differential regulation of serotonin (5HT)2A receptor mRNA and protein levels after single and repeated stress in rat brain: role in learned helplessness behavior. Neuropharmacology. 2005;48:204–14.
Lopez JF, Chalmers DT, Little KY, Watson SJ. A. E. Bennett Research Award. Regulation of serotonin 1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: Implications for the neurobiology of depression. Biol Psychiatry 1998;43:547–73.
McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37:383–93.
Watanabe Y, Sakai RR, McEwen BS, Mendelson S. Stress and antidepressant effects on hippocampal and cortical 5-HT1A and 5-HT2 receptors and transport sites for serotonin. Brain Res. 1993;615:87–94.
Bettinardi V, Castiglioni I, De Bernardi E, Gilardi MC. PET quantification: strategies for partial volume correction. Clin Transl Imaging. 2014;2:199–218.
Rousset O, Rahmim A, Alavi A, Zaidi H. Partial volume correction strategies in PET. PET Clin. 2007;2:235–49.
Hoetjes NJ, van Velden FH, Hoekstra OS, Hoekstra CJ, Krak NC, Lammertsma AA, et al. Partial volume correction strategies for quantitative FDG PET in oncology. Eur J Nucl Med Mol Imaging. 2010;37:1679–87.
Tohka J, Reilhac A. Deconvolution-based partial volume correction in Raclopride-PET and Monte Carlo comparison to MR-based method. NeuroImage. 2008;39:1570–84.
Carson RE, Channing MA, Blasberg RG, Dunn BB, Cohen RM, Rice KC, et al. Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993;13:24–42.
Koeppe RA, Frey KA, Mulholland GK, Kilbourn MR, Buck A, Lee KS, et al. [11C]tropanyl benzilate-binding to muscarinic cholinergic receptors: methodology and kinetic modeling alternatives. J Cereb Blood Flow Metab. 1994;14:85–99.
Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol. 2000;27:661–70.
Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–112.
Ewing Corcoran SB, Howell LL. Impact of early life stress on the reinforcing and behavioral-stimulant effects of psychostimulants in rhesus monkeys. Behav Pharm. 2010;21:69–76.
Muly EC, Votaw JR, Ritchie J, Howell LL. Relationship between dose, drug levels, and D2 receptor occupancy for the atypical antipsychotics risperidone and paliperidone. J Pharm Exp Ther. 2012;341:81–9.
Sawyer EK, Mun J, Nye JA, Kimmel HL, Voll RJ, Stehouwer JS, et al. Neurobiological changes mediating the effects of chronic fluoxetine on cocaine use. Neuropsychopharmacology. 2012;37:1816–24.
Voisin DA, Wakeford A, Nye J, Mun J, Jones SR, Locke J, et al. Sex and social status modify the effects of fluoxetine on socioemotional behaviors in Syrian hamsters and rhesus macaques. Pharm Biochem Behav. 2022;215:173362.
Acknowledgements
These data were reported in abstract form at the American College for Neuropsychopharmacology in 2018, as well as the College on Problems of Drug Dependence in 2018. The authors want to thank Brittany Howell, Anne Glenn, Christine Marsteller, Dora Guzman, Erin Siebert, and the staff at the Emory National Primate Research Center (ENPRC) for their contributions, excellent technical support and animal care provided during these studies. In addition, we thank Dr. Melinda Higgins for Biostatistical guidance and support.
Funding
This work was supported by funding from NIH/NIDA grant DA038588 and NIH/NIMH grant MH078105, as well as by the Emory National Primate Research Center (ENPRC) grant No. ORIP/OD P51OD011132 (ENPRC Base grant; the ENPRC is supported by the NIH, Office of Research Infrastructure Programs/OD [P51OD011132]) and by the Emory HPLC Bioanalytical Core (EHBC, which is supported by the Department of Pharmacology, Emory University School of Medicine and the Georgia Clinical & Translational Science Alliance of the NIH under Award Number UL1TR002378). The funders had no role in review design, data collection and analysis, decision to publish, or preparation of the manuscript; the content is solely the responsibility of the authors and does not represent the official views of the NIDA, NIMH or the NIH. The ENPRC is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, AAALAC, International.
Author information
Authors and Affiliations
Contributions
AGPW collected data, conducted analyses, prepared figures, and wrote the manuscript. ELM, JM, and JSM conducted analyses and helped with manuscript preparation. JAN conducted analyses, designed part of these experiments, and revised this manuscript. MG, LLH, and MMS helped to design the experiment and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wakeford, A.G.P., Nye, J.A., Morin, E.L. et al. Alterations in adolescent brain serotonin (5HT)1A, 5HT2A, and dopamine (D)2 receptor systems in a nonhuman primate model of early life adversity. Neuropsychopharmacol. (2024). https://doi.org/10.1038/s41386-023-01784-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41386-023-01784-0