Abstract
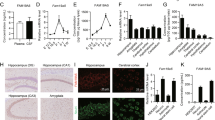
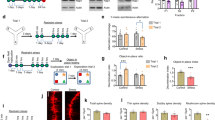
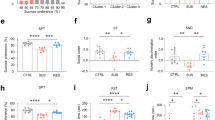
Trace amine-associated receptor 1 (TAAR1) is an intracellular expressed G-protein-coupled receptor that is widely expressed in major dopaminergic areas and plays a crucial role in modulation of central dopaminergic neurotransmission and function. Pharmacological studies have clarified the roles of dopamine D1 receptor (D1R) in the medial prefrontal cortex (mPFC) in cognitive function and social behaviors, and chronic stress can inhibit D1R expression due to its susceptibility. Recently, we identified TAAR1 in the mPFC as a potential target for treating chronic stress-induced cognitive and social dysfunction, but whether D1R is involved in mediating the effects of TAAR1 agonist remains unclear. Combined genomics and transcriptomic studies revealed downregulation of D1R in the mPFC of TAAR1−/− mice. Molecular dynamics simulation showed that hydrogen bond, salt bridge, and Pi–Pi stacking interactions were formed between TAAR1 and D1R indicating a stable TAAR1–D1R complex structure. Using pharmacological interventions, we found that D1R antagonist disrupted therapeutic effect of TAAR1 partial agonist RO5263397 on stress-related cognitive and social dysfunction. Knockout TAAR1 in D1-type dopamine receptor-expressing neurons reproduced adverse effects of chronic stress, and TAAR1 conditional knockout in the mPFC led to similar deficits, along with downregulation of D1R expression, all of these effects were ameliorated by viral overexpression of D1R in the mPFC, suggesting the functional interaction between TAAR1 and D1R. Collectively, our data elucidate the possible molecular mechanism that D1R in the mPFC mediates the effects of TAAR1 activation on chronic stress-induced cognitive and social deficits.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Miller GM, Verrico CD, Jassen A, Konar M, Yang H, Panas H, et al. Primate trace amine receptor 1 modulation by the dopamine transporter. J Pharmacol Exp Ther. 2005;313:983–94.
Revel FG, Meyer CA, Bradaia A, Jeanneau K, Calcagno E, Andre CB, et al. Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine. Neuropsychopharmacology. 2012;37:2580–92.
Dorotenko A, Tur M, Dolgorukova A, Bortnikov N, Belozertseva IV, Zvartau EE, et al. The action of TAAR1 agonist RO5263397 on executive functions in rats. Cell Mol Neurobiol. 2020;40:215–28.
Wu R, Liu J, Seaman R Jr, Johnson B, Zhang Y, Li JX. The selective TAAR1 partial agonist RO5263397 promoted novelty recognition memory in mice. Psychopharmacology. 2021;238:3221–8.
Tanaka K, Furuyashiki T, Kitaoka S, Senzai Y, Imoto Y, Segi-Nishida E, et al. Prostaglandin E2-mediated attenuation of mesocortical dopaminergic pathway is critical for susceptibility to repeated social defeat stress in mice. J Neurosci. 2012;32:4319–29.
Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45.
Tzschentke TM. The medial prefrontal cortex as a part of the brain reward system. Amino Acids. 2000;19:211–9.
Cools R, Arnsten AFT. Neuromodulation of prefrontal cortex cognitive function in primates: the powerful roles of monoamines and acetylcholine. Neuropsychopharmacology. 2022;47:309–28.
Xu P, Yue YL, Su JT, Sun XQ, Du HF, Liu ZC, et al. Pattern decorrelation in the mouse medial prefrontal cortex enables social preference and requires MeCP2. Nat Commun. 2022;13:3899.
Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7.
Tzschentke TM. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol. 2001;63:241–320.
Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217.
Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology. 2004;174:3–16.
Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–39.
Xing B, Mack NR, Guo KM, Zhang YX, Ramirez B, Yang SS, et al. A subpopulation of prefrontal cortical neurons is required for social memory. Biol Psychiatry. 2021;89:521–31.
Cai XW, Wu M, Zhang ZA, Liu HC, Huang ST, Song J, et al. Electroacupuncture alleviated depression-like behaviors in ventromedial prefrontal cortex of chronic unpredictable mild stress-induced rats: increasing synaptic transmission and phosphorylating dopamine transporter. CNS Neurosci Ther. 2023;29:2608–20.
Shinohara R, Taniguchi M, Ehrlich AT, Yokogawa K, Deguchi Y, Cherasse Y, et al. Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol Psychiatry. 2018;23:1717–30.
Zhang Y, Li JT, Wang H, Niu WP, Zhang CC, Zhang Y, et al. Role of trace amine‑associated receptor 1 in the medial prefrontal cortex in chronic social stress-induced cognitive deficits in mice. Pharmacol Res. 2021;167:105571.
Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front Neurosci. 2016;10:148.
Xie ZH, Miller GM. Trace amine-associated receptor 1 as a monoaminergic modulator in brain. Biochem Pharmacol. 2009;78:1095–104.
Xie Z, Westmoreland SV, Miller GM. Modulation of monoamine transporters by common biogenic amines via trace amine-associated receptor 1 and monoamine autoreceptors in human embryonic kidney 293 cells and brain synaptosomes. J Pharmacol Exp Ther. 2008;325:629–40.
Harmeier A, Obermueller S, Meyer CA, Revel FG, Buchy D, Chaboz S, et al. Trace amine-associated receptor 1 activation silences GSK3β signaling of TAAR1 and D2R heteromers. Eur Neuropsychopharm. 2015;25:2049–61.
Espinoza S, Salahpour A, Masri B, Sotnikova TD, Messa M, Barak LS, et al. Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol Pharmacol. 2011;80:416–25.
Leo D, Mus L, Espinoza S, Hoener MC, Sotnikova TD, Gainetdinov RR. TAAR1-mediated modulation of presynaptic dopaminergic neurotransmission: role of D2 dopamine autoreceptors. Neuropharmacology. 2014;81:283–91.
De Gregorio D, Posa L, Ochoa-Sanchez R, McLaughlin R, Maione S, Comai S, et al. The hallucinogen D-lysergic diethylamide (LSD) decreases dopamine firing activity through 5-HT1A, D2 and TAAR1 receptors. Int J Neuropsychoph. 2016;19:181.
Grinchii D, Hoener MC, Khoury T, Dekhtiarenko R, Bervanlou RN, Jezova D, et al. Effects of acute and chronic administration of trace amine-associated receptor 1 (TAAR1) ligands on in vivo excitability of central monoamine-secreting neurons in rats. Mol Psychiatry. 2022;27:4861–8.
Espinoza S, Leou D, Sotnikova TD, Shahid M, Kääriäinen TM, Gainetdinov RR. Biochemical and functional characterization of the trace amine-associated receptor 1 (TAAR1) agonist RO5263397. Front Pharmacol. 2018;9:645.
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410.
Wagner KV, Wang XD, Liebl C, Scharf SH, Müller MB, Schmidt MV. Pituitary glucocorticoid receptor deletion reduces vulnerability to chronic stress. Psychoneuroendocrinology. 2011;36:579–87.
Haenisch B, Bilkei-Gorzo A, Caron MG, Bönisch H. Knockout of the norepinephrine transporter and pharmacologically diverse antidepressants prevent behavioral and brain neurotrophin alterations in two chronic stress models of depression. J Neurochem. 2009;111:403–16.
Li JT, Xie XM, Yu JY, Sun YX, Liao XM, Wang XX, et al. Suppressed calbindin levels in hippocampal excitatory neurons mediate stress-induced memory loss. Cell Rep. 2017;21:891–900.
Yang XD, Liao XM, Uribe-Marino A, Liu R, Xie XM, Jia J, et al. Stress during a critical postnatal period induces region-specific structural abnormalities and dysfunction of the prefrontal cortex via CRF1. Neuropsychopharmacology. 2015;40:1203–15.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303.
Biasini M, Schmidt T, Bienert S, Mariani V, Studer G, Haas J, et al. OpenStructure: an integrated software framework for computational structural biology. Acta Crystallogr D Biol Crystallogr. 2013;69:701–9.
Yan Y, Tao H, He J, Huang SY. The HDOCK server for integrated protein-protein docking. Nat Protoc. 2020;15:1829–52.
Lundborg M, Lindahl E. Automatic GROMACS topology generation and comparisons of force fields for solvation free energy calculations. J Phys Chem B. 2015;119:810–23.
Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE, Simmerling C. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J Chem Theory Comput. 2015;11:3696–713.
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–35.
Berendsen HJC, Postma JPM, Vangunsteren WF, Dinola A, Haak JR. Molecular-dynamics with coupling to an external bath. J Chem Phys. 1984;81:3684–90.
Parrinello M, Rahman A. Polymorphic transitions in single-crystals – a new molecular-dynamics method. J Appl Phys. 1981;52:7182–90.
Valdés-Tresanco MS, Valdés-Tresanco ME, Valiente PA, Moreno E. gmx_MMPBSA: a new tool to perform end-state free energy calculations with GROMACS. J Chem Theory Comput. 2021;17:6281–91.
Miller BR, McGee TD, Swails JM, Homeyer N, Gohlke H, Roitberg AE. MMPBSA.py: an efficient program for end-state free energy calculations. J Chem Theory Comput. 2012;8:3314–21.
Rutigliano G, Accorroni A, Zucchi R. The case for TAAR1 as a modulator of central nervous system function. Front Pharmacol. 2018;8:987.
Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, et al. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci USA 2009;106:20081–6.
Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 2007;65:209–37.
Marin MF, Lord C, Andrews J, Juster RP, Sindi S, Arsenault-Lapierre G, et al. Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem. 2011;96:583–95.
Belleau EL, Treadway MT, Pizzagalli DA. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry. 2019;85:443–53.
Sun MK, Alkon DL. Stress: perspectives on its impact on cognition and pharmacological treatment. Behav Pharmacol. 2014;25:410–24.
Arnsten AFT, Wang M, Paspalas CD. Dopamine’s actions in primate prefrontal cortex: challenges for treating cognitive disorders. Pharmacol Rev. 2015;67:681–96.
Arnsten AFT. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci. 2015;18:1376–85.
Li Q, Zhang B, Cao H, Liu W, Guo F, Shen F, et al. Oxytocin exerts antidepressant-like effect by potentiating dopaminergic synaptic transmission in the mPFC. Neuropharmacology. 2020;162:107836.
Ostroumov A, Dani JA. Inhibitory plasticity of mesocorticolimbic circuits in addiction and mental illness. Trends Neurosci. 2018;41:898–910.
Fortier AV, Meisner OC, Nair AR, Chang SWC. Prefrontal circuits guiding social preference: implications in autism spectrum disorder. Neurosci Biobehav Rev. 2022;141:104803.
Revel FG, Moreau JL, Pouzet B, Mory R, Bradaia A, Buchy D, et al. A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry. 2013;18:543–56.
Rutigliano G, Zucchi R. Molecular variants in human trace amine-associated receptors and their implications in mental and metabolic disorders. Cell Mol Neurobiol. 2020;40:239–55.
Arnsten AF, Girgis RR, Gray DL, Mailman RB. Novel dopamine therapeutics for cognitive deficits in schizophrenia. Biol Psychiatry. 2017;81:67–77.
Puig MV, Miller EK. The role of prefrontal dopamine D1 receptors in the neural mechanisms of associative learning. Neuron. 2012;74:874–86.
Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–6.
Pignatelli M, Tejeda HA, Barker DJ, Bontempi L, Wu JC, Lopez A, et al. Cooperative synaptic and intrinsic plasticity in a disynaptic limbic circuit drive stress-induced anhedonia and passive coping in mice. Mol Psychiatry. 2021;26:1860–79.
Gainetdinov RR, Hoener MC, Berry MD. Trace amines and their receptors. Pharmacol Rev. 2018;70:549–620.
Berry MD, Gainetdinov RR, Hoener MC, Shahid M. Pharmacology of human trace amine-associated receptors: therapeutic opportunities and challenges. Pharmacol Ther. 2017;180:161–80.
Raab S, Wang H, Uhles S, Cole N, Alvarez-Sanchez R, Kunnecke B, et al. Incretin-like effects of small molecule trace amine-associated receptor 1 agonists. Mol Metab. 2016;5:47–56.
Xu Z, Guo L, Yu J, Shen S, Wu C, Zhang W, et al. Ligand recognition and G-protein coupling of trace amine receptor TAAR1. Nature. 2023;624:672–81.
Funding
This work was supported by the National Natural Science Foundation of China (82071528, 81771468, 82171529, 82271569).
Author information
Authors and Affiliations
Contributions
Meng Sun: investigation, formal analysis, methodology, writing – original draft. Yue Zhang: investigation, formal analysis, methodology, writing – original draft. Xian-Qiang Zhang: investigation, formal analysis, methodology. Yanan Zhang: resources, investigation. Xiao-Dong Wang: conceptualization, investigation. Ji-Tao Li: conceptualization, investigation, supervision, writing – review & editing. Tian-Mei Si: conceptualization, funding acquisition, supervision, writing – review & editing. Yun-Ai Su: conceptualization, funding acquisition, investigation, supervision, writing – review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, M., Zhang, Y., Zhang, XQ. et al. Dopamine D1 receptor in medial prefrontal cortex mediates the effects of TAAR1 activation on chronic stress-induced cognitive and social deficits. Neuropsychopharmacol. (2024). https://doi.org/10.1038/s41386-024-01866-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41386-024-01866-7