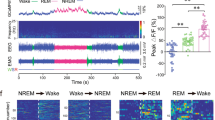
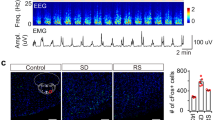
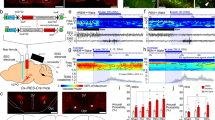
Abstract
The ventral subiculum (vSUB), the major output structure of the hippocampal formation, regulates motivation, stress integration, and anxiety-like behaviors that rely on heightened arousal. However, the roles and underlying neural circuits of the vSUB in wakefulness are poorly known. Using in vivo fiber photometry and multichannel electrophysiological recordings in mice, we found that the vSUB glutamatergic neurons exhibited high activities during wakefulness. Moreover, activation of vSUB glutamatergic neurons caused an increase in wakefulness and anxiety-like behaviors and induced a rapid transition from sleep to wakefulness. In addition, optogenetic stimulation of vSUB glutamatergic terminals and retrograde-targeted chemogenetic activation of vSUB glutamatergic neurons revealed that vSUB promoted arousal by innervating the lateral hypothalamus (LH), nucleus accumbens (NAc) shell, and prefrontal cortex (PFC). Nevertheless, local microinjection of dopamine D1 or D2/D3 receptor antagonist blocked the wake-promoting effect induced by chemogenetic activation of vSUB pathways. Finally, chemogenetic inhibition of vSUB glutamatergic neurons decreased arousal. Altogether, our findings reveal a prominent contribution of vSUB glutamatergic neurons to the control of wakefulness through several pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The raw data generated in this study have been deposited in the Figshare database under accession code: https://doi.org/10.6084/m9.figshare.25658037.
References
Mueller NK, Dolgas CM, Herman JP. Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology. 2004;145:3763–68.
Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19.
Yan JJ, Ding XJ, He T, Chen AX, Zhang W, Yu ZX, et al. A circuit from the ventral subiculum to anterior hypothalamic nucleus GABAergic neurons essential for anxiety-like behavioral avoidance. Nat Commun. 2022;13:7464.
Sun YJ, Jin SQ, Lin XX, Chen LJ, Qiao X, Jiang L, et al. CA1-projecting subiculum neurons facilitate object–place learning. Nat Neurosci. 2019;22:1857–70.
Torromino G, Autore L, Khalil V, Mastrorilli V, Griguoli M, Pignataro A, et al. Offline ventral subiculum-ventral striatum serial communication is required for spatial memory consolidation. Nat Commun. 2019;10:5721.
Grimm CM, Aksamaz S, Schulz S, Teutsch J, Sicinski P, Liss B, et al. Schizophrenia-related cognitive dysfunction in the Cyclin-D2 knockout mouse model of ventral hippocampal hyperactivity. Transl Psychiatry. 2018;8:212.
Meyer FF, Louilot A. Latent inhibition-related dopaminergic responses in the nucleus accumbens are disrupted following neonatal transient inactivation of the ventral subiculum. Neuropsychopharmacology. 2011;36:1421–32.
Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47.
Sun WL, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–64.
Glangetas C, Fois GR, Jalabert M, Lecca S, Valentinova K, Meye FJ, et al. Ventral subiculum stimulation promotes persistent hyperactivity of dopamine neurons and facilitates behavioral effects of cocaine. Cell Rep. 2015;13:2287–96.
Spiegelhalder K, Regen W, Nanovska S, Baglioni C, Riemann D. Comorbid sleep disorders in neuropsychiatric disorders across the life cycle. Curr Psychiatry Rep. 2013;15:364.
Richard JS, Charles LW, Anatol B, Itzhak F, Engel J,Jr. Sleep states differentiate single neuron activity recorded from human epileptic hippocampus, entorhinal cortex, and subiculum. J Neurosci. 2002;22:5694
Hagan JJ, Verheijck EE, Spigt MH, Ruigt GSF. Behavioural and electrophysiological studies of entorhinal cortex lesions in the rat. Physiol Behav. 1992;51:255–66.
Macey PM, Prasad JP, Ogren JA, Moiyadi AS, Aysola RS, Kumar R, et al. Sex-specific hippocampus volume changes in obstructive sleep apnea. Neuroimage Clin. 2018;20:305–17.
De Looze C, Feeney JC, Scarlett S, Hirst R, Knight SP, Carey D, et al. Sleep duration, sleep problems, and perceived stress are associated with hippocampal subfield volumes in later life: findings from The Irish Longitudinal Study on Ageing. Sleep. 2022;45:zsab241.
Bienkowski MS, Bowman I, Song MY, Gou L, Ard T, Cotter K, et al. Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks. Nat Neurosci. 2018;21:1628–43.
Wee RWS, MacAskill AF. Biased connectivity of brain-wide inputs to ventral subiculum output neurons. Cell Rep. 2020;30:3644–54.
O’Mara SM, Commins S, Anderson M, Gigg J. The subiculum: a review of form, physiology and function. Prog Neurobiol. 2001;64:129–55.
Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–22.
Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803.
Marek R, Jin J, Goode TD, Giustino TF, Wang Q, Acca GM, et al. Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat Neurosci. 2018;21:384–92.
Hsu TM, Hahn JD, Konanur VR, Noble EE, Suarez AN, Thai J, et al. Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. eLife. 2015;4:e11190.
Peleg-Raibstein D, Feldon J. Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology. 2006;51:947–57.
Taepavarapruk P, Howland JG, Ahn S, Phillips AG. Neural circuits engaged in ventral hippocampal modulation of dopamine function in medial prefrontal cortex and ventral striatum. Brain Struct Funct. 2008;213:183–95.
Léna I, Parrot S, Deschaux O, Muffat-Joly S, Sauvinet V, Renaud B, et al. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep–wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81:891–99.
Luo YJ, Li YD, Wang L, Yang SR, Yuan XS, Wang J, et al. Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors. Nat Commun. 2018;9:1576.
Justinussen JL, Egebjerg C, Kornum BR. How hypocretin agonists may improve the quality of wake in narcolepsy. Trends Mol. Med. 2023;29:61–69.
Yamashita T, Yamanaka A. Lateral hypothalamic circuits for sleep–wake control. Curr Opin Neurobiol. 2017;44:94–100.
Mashour GA, Pal D, Brown EN. Prefrontal cortex as a key node in arousal circuitry. Trends Neurosci. 2022;45:722–32.
Rahimi S, Joyce L, Fenzl T, Drexel M. Crosstalk between the subiculum and sleep–wake regulation: a review. J. Sleep Res. 2024:e14134. https://doi.org/10.1111/jsr.14134. Online ahead of print.
Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–187.
Eban-Rothschild A, Appelbaum L, de Lecea L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology. 2018;43:937–52.
Sulaman BA, Wang S, Tyan J, Eban-Rothschild A. Neuro-orchestration of sleep and wakefulness. Nat Neurosci. 2023;26:196–212.
Rayan A, Agarwal A, Samanta A, Severijnen E, van der Meij J, Genzel L. Sleep scoring in rodents: Criteria, automatic approaches and outstanding issues. Eur J Neurosci. 2022;59:526–553.
Traut J, Mengual JP, Meijer EJ, McKillop LE, Alfonsa H, Hoerder-Suabedissen A, et al. Effects of clozapine-N-oxide and compound 21 on sleep in laboratory mice. eLife. 2023;12:e84740.
Urien L, Cohen S, Howard S, Yakimov A, Nordlicht R, Bauer EP. Aversive contexts reduce activity in the ventral subiculum- BNST pathway. Neuroscience. 2022;496:129–40.
Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 2015;66:25–52.
Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–69.
Schoenfeld TJ, Kloth AD, Hsueh B, Runkle MB, Kane GA, Wang SS-H, et al. Gap junctions in the ventral hippocampal-medial prefrontal pathway are involved in anxiety regulation. J Neurosci. 2014;34:15679–88.
Parfitt GM, Nguyen R, Bang JY, Aqrabawi AJ, Tran MM, Seo DK, et al. Bidirectional control of anxiety-related behaviors in mice: role of inputs arising from the ventral hippocampus to the lateral septum and medial prefrontal cortex. Neuropsychopharmacology. 2017;42:1715–28.
Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron. 2016;89:857–66.
Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–64.
Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, et al. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron. 2018;97:670–83.
Adhikari A, Topiwala MA, Gordon JA. Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron. 2011;71:898–910.
Cornwell BR, Arkin N, Overstreet C, Carver FW, Grillon C. Distinct contributions of human hippocampal theta to spatial cognition and anxiety. Hippocampus. 2012;22:1848–59.
Bannerman DM, Deacon RMJ, Offen S, Friswell J, Grubb M, Rawlins JNP. Double dissociation of function within the hippocampus: Spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901.
Arrigoni E, Chee MJS, Fuller PM. To eat or to sleep: that is a lateral hypothalamic question. Neuropharmacology. 2019;154:34–49.
Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20.
Tsunematsu T, Ueno T, Tabuchi S, Inutsuka A, Tanaka KF, Hasuwa H, et al. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci. 2014;34:6896–909.
Vetrivelan R, Kong D, Ferrari LL, Arrigoni E, Madara JC, Bandaru SS, et al. Melanin-concentrating hormone neurons specifically promote rapid eye movement sleep in mice. Neuroscience. 2016;336:102–13.
Hahn JD, Swanson LW. Connections of the juxtaventromedial region of the lateral hypothalamic area in the male rat. Front Syst Neurosci. 2015;9:66.
Hahn JD, Swanson LW. Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain Res Rev. 2010;64:14–103.
Folgueira C, Beiroa D, Porteiro B, Duquenne M, Puighermanal E, Fondevila MF, et al. Hypothalamic dopamine signalling regulates brown fat thermogenesis. Nat. Metab. 2019;1:811–29.
Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–90.
Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep–wake behaviors. Nat Neurosci. 2016;19:1356–66.
Baimel C, McGarry LM, Carter AG. The projection targets of medium spiny neurons govern cocaine-evoked synaptic plasticity in the nucleus accumbens. Cell Rep. 2019;28:2256–63.
Zinsmaier AK, Dong Y, Huang YH. Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens. Mol Psychiatry. 2022;27:669–86.
Oishi Y, Suzuki Y, Takahashi K, Yonezawa T, Kanda T, Takata Y, et al. Activation of ventral tegmental area dopamine neurons produces wakefulness through dopamine D2-like receptors in mice. Brain Struct Funct. 2017;222:2907–15.
Briand LA, Gritton H, Howe WM, Young DA, Sarter M. Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Prog Neurobiol. 2007;83:69–91.
Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79.
Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. J Neurosci. 2003;23:3930–43.
Ott T, Nieder A. Dopamine and cognitive control in prefrontal cortex. Trends Cogn Sci. 2019;23:213–34.
Li YD, Luo YJ, Xu W, Ge J, Cherasse Y, Wang YQ, et al. Ventral pallidal GABAergic neurons control wakefulness associated with motivation through the ventral tegmental pathway. Mol Psychiatry. 2021;26:2912–28.
Han Y, Shi YF, Xi W, Zhou R, Tan ZB, Wang H, et al. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr. Biol. 2014;24:693–98.
Boucetta S, Cissé Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep–waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34:4708.
Ren SC, Wang YL, Yue FG, Cheng XF, Dang RZ, Qiao QC, et al. The paraventricular thalamus is a critical thalamic area for wakefulness. Science. 2018;362:429–34.
Fei F, Wang X, Xu C, Shi J, Gong Y, Cheng H, et al. Discrete subicular circuits control generalization of hippocampal seizures. Nat Commun. 2022;13:5010.
Swift KM, Keus K, Echeverria CG, Cabrera Y, Jimenez J, Holloway J, et al. Sex differences within sleep in gonadally intact rats. Sleep. 2020;43:zsz289.
Bagot RC, Parise EM, Peña CJ, Zhang HX, Maze I, Chaudhury D, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062.
Acknowledgements
We thank the following individuals for technical assistance to this work: Xiao Tan, Yuyang Ni, Meifang Ma, Zhenzhen Tian, Caijun Dong, Shuping Fang, Wenting Yang, Hongjun Liu, Feiyang Zhang, Yun Wang, Wei Jing.
Funding
This study was supported by National Natural Science Foundation of China (grants: 81771819 and 32071140), the Scientific Research Project of Hubei Provincial Health Commission (grant: WJ2019H058), the Scientific Research Project of Traditional Chinese Medicine of Hubei Province (ZY2023Q032) and Research Grant of Guangdong Province Key Laboratory of Psychiatric Disorders (N202301).
Author information
Authors and Affiliations
Contributions
X-.FZ, H-.BX and L-LB designed the experiments. X-FZ, Y-DL, Yue Li, Ying Li and DX collected and analysed the data. X-FZ, and Y-DL discussed the results and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, XF., Li, YD., Li, Y. et al. Ventral subiculum promotes wakefulness through several pathways in male mice. Neuropsychopharmacol. (2024). https://doi.org/10.1038/s41386-024-01875-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41386-024-01875-6