Abstract
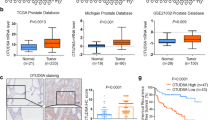
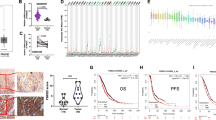
Receptor-interacting protein kinase 4 (RIPK4) is increasingly recognized as a pivotal player in ovarian cancer, promoting tumorigenesis and disease progression. Despite its significance, the posttranslational modifications dictating RIPK4 stability in ovarian cancer remain largely uncharted. In this study, we first established that RIPK4 levels are markedly higher in metastatic than in primary ovarian cancer tissues through single-cell sequencing. Subsequently, we identified UCHL3 as a key deubiquitinase that regulates RIPK4. We elucidate the mechanism that UCHL3 interacts with and deubiquitinates RIPK4 at the K469 site, removing the K48-linked ubiquitin chain and thus enhancing RIPK4 stabilization. Intriguingly, inhibition of UCHL3 activity using TCID leads to increased RIPK4 ubiquitination and degradation. Furthermore, we discovered that GSK3β-mediated phosphorylation of RIPK4 at Ser420 enhances its interaction with UCHL3, facilitating further deubiquitination and stabilization. Functionally, RIPK4 was found to drive the proliferation and metastasis of ovarian cancer in a UCHL3-dependent manner both in vitro and in vivo. Importantly, positive correlations between RIPK4 and UCHL3 protein expression levels were observed, with both serving as indicators of poor prognosis in ovarian cancer patients. Overall, this study uncovers a novel pathway wherein GSK3β-induced phosphorylation of RIPK4 strengthens its interaction with UCHL3, leading to increased deubiquitination and stabilization of RIPK4, thereby promoting ovarian cancer metastasis. These findings offer new insights into the molecular underpinnings of ovarian cancer and highlight potential therapeutic targets for enhancing antitumor efficacy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Some data analyzed in this study were obtained from the Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA). The human sequence data generated in this study are not publicly available because of patient privacy requirements but are available from the corresponding author upon reasonable request.
References
El-Arabey AA, Alkhalil SS, Al-Shouli ST, Awadalla ME, Alhamdi HW, Almanaa TN, et al. Revisiting macrophages in ovarian cancer microenvironment: development, function, and interaction. Med Oncol. 2023;40:142.
Mei S, Chen X, Wang K, Chen Y. Tumor microenvironment in ovarian cancer peritoneal metastasis. Cancer Cell Int. 2023;23:11.
Tossetta G, Inversetti A. Ovarian cancer: advances in pathophysiology and therapies. Int J Mol Sci. 2023;24:8930.
Shu F, Xiao H, Li QN, Ren XS, Liu ZG, Hu BW, et al. Epigenetic and post-translational modifications in autophagy: biological functions and therapeutic targets. Signal Transduct Target Ther. 2023;8:32.
Wang H, Yang L, Liu M, Luo J. Protein post-translational modifications in the regulation of cancer hallmarks. Cancer Gene Ther. 2023;30:529–47.
Zhong Q, Xiao X, Qiu Y, Xu Z, Chen C, Chong B, et al. Protein posttranslational modifications in health and diseases: functions, regulatory mechanisms, and therapeutic implications. MedComm. 2023;4:e261.
Zhao Z, Xu H, Wei Y, Sun L, Song Y. Deubiquitylase PSMD14 inhibits autophagy to promote ovarian cancer progression via stabilization of LRPPRC. Biochim Biophys Acta Mol Basis Dis. 2023;1869:166594.
Pan S, Chen R. Pathological implication of protein post-translational modifications in cancer. Mol Aspects Med. 2022;86:101097.
Ren C, Han X, Lu C, Yang T, Qiao P, Sun Y, et al. Ubiquitination of NF-κB p65 by FBXW2 suppresses breast cancer stemness, tumorigenesis, and paclitaxel resistance. Cell Death Differ. 2022;29:381–92.
Xiao X, Shi J, He C, Bu X, Sun Y, Gao M, et al. ERK and USP5 govern PD-1 homeostasis via deubiquitination to modulate tumor immunotherapy. Nat Commun. 2023;14:2859.
Peng Y, Liu J, Inuzuka H, Wei W. Targeted protein posttranslational modifications by chemically induced proximity for cancer therapy. J Biol Chem. 2023;299:104572.
Li G, Xu Z, Peng J, Yan Y, Liu Y, Zhang X, et al. The RIPK family: expression profile and prognostic value in lung adenocarcinoma. Aging. 2022;14:5946–58.
Huang FY, Dai SZ, Xu WT, Xiong W, Sun Y, Huang YH, et al. 3’-epi-12β-hydroxyfroside-mediated autophagy degradation of RIPK1/RIPK3 necrosomes leads to anergy of immunogenic cell death in triple-negative breast cancer cells. Pharmacol Res. 2023;187:106613.
Liao C, Zhao YX, Han WD, Lai NY. RIPK4 is an immune regulating-associated biomarker for ovarian cancer and possesses generalization value in pan-cancer. J Immunol Res. 2022;2022:7599098.
Zhong GY, Tan JN, Huang J, Zhou SN, Yu JH, Zhong L, et al. LncRNA LINC01537 promotes gastric cancer metastasis and tumorigenesis by stabilizing RIPK4 to activate NF-κB signaling. Cancers. 2022;14:5237.
Jin A, Zhang L, Fang G, Chen Y. Receptor interacting protein kinase 4 promotes cell proliferation, migration, and invasion in ovarian cancer via targeting protein kinase C delta. Drug Dev Res. 2022;83:407–15.
Yan Y, Gauthier MA, Malik A, Fotiadou I, Ostrovski M, Dervovic D, et al. The NOTCH-RIPK4-IRF6-ELOVL4 axis suppresses squamous cell carcinoma. Cancers. 2023;15:737.
Pan WY, Zeng JH, Wen DY, Wang JY, Wang PP, Chen G, et al. Oncogenic value of microRNA-15b-5p in hepatocellular carcinoma and a bioinformatics investigation. Oncol Lett. 2019;17:1695–713.
Cai L, Ye L, Hu X, He W, Zhuang D, Guo Q, et al. MicroRNA miR-330-3p suppresses the progression of ovarian cancer by targeting RIPK4. Bioengineered. 2021;12:440–9.
Zhou QQ, Xiao HT, Yang F, Wang YD, Li P, Zheng ZG. Advancing targeted protein degradation for metabolic diseases therapy. Pharmacol Res. 2023;188:106627.
Nakamura N. Ubiquitin system. Int J Mol Sci. 2018;19:1080.
Ren Y, Feng M, Hao X, Liu X, Li J, Li P, et al. USP48 stabilizes gasdermin e to promote pyroptosis in cancer. Cancer Res. 2023;83:1074–93.
Tang J, Yang Q, Mao C, Xiao D, Liu S, Xiao L, et al. The deubiquitinating enzyme UCHL3 promotes anaplastic thyroid cancer progression and metastasis through Hippo signaling pathway. Cell Death Differ. 2023;30:1247–59.
Ouyang L, Yan B, Liu Y, Mao C, Wang M, Liu N, et al. The deubiquitylase UCHL3 maintains cancer stem-like properties by stabilizing the aryl hydrocarbon receptor. Signal Transduct Target Ther. 2020;5:78.
Barbour H, Nkwe NS, Estavoyer B, Messmer C, Gushul-Leclaire M, Villot R, et al. An inventory of crosstalk between ubiquitination and other post-translational modifications in orchestrating cellular processes. iScience. 2023;26:106276.
Hu L, Liu M, Tang B, Li Q, Pan BS, Xu C, et al. Posttranslational regulation of liver kinase B1 in human cancer. J Biol Chem. 2023;299:104570.
Khilar P, Sruthi KK, Parveen SMA, Natani S, Jadav SS, Ummanni R. AMPK targets a proto-oncogene TPD52 (isoform 3) expression and its interaction with LKB1 suppress AMPK-GSK3β signaling axis in prostate cancer. J Cell Commun Signal. 2023;17:957–74.
Yi Z, Pu Y, Gou R, Chen Y, Ren X, Liu W, et al. Silencing of RIPK4 inhibits epithelial‑mesenchymal transition by inactivating the Wnt/β‑catenin signaling pathway in osteosarcoma. Mol Med Rep. 2020;21:1154–62.
Liu S, He L, Sheng C, Su R, Wu X, Sun Y, et al. Overexpression of RIPK4 predicts poor prognosis and promotes metastasis in ovarian cancer. BioMed Res Int. 2021;2021:6622439.
Ren Y, Yu B, Zhou L, Wang F, Wang Y. Structural Insights into the phosphorylation-enhanced deubiquitinating activity of UCHL3 and ubiquitin chain cleavage preference analysis. Int J Mol Sci. 2022;23:10789.
Zhang Y, Liu JB, Liu J, Liu M, Liu HL, Zhang J. UCHL3 promotes cervical cancer development and metastasis by stabilizing NRF2 via deubiquitination. Biochem Biophys Res Commun. 2023;641:132–8.
Zhang MH, Zhang HH, Du XH, Gao J, Li C, Shi HR, et al. UCHL3 promotes ovarian cancer progression by stabilizing TRAF2 to activate the NF-κB pathway. Oncogene. 2020;39:322–33.
Luo K, Li L, Li Y, Wu C, Yin Y, Chen Y, et al. A phosphorylation-deubiquitination cascade regulates the BRCA2-RAD51 axis in homologous recombination. Genes Dev. 2016;30:2581–95.
Liu L, Cai L, Liu C, Yu S, Li B, Pan L, et al. Construction and validation of a novel glycometabolism-related gene signature predicting survival in patients with ovarian cancer. Front Genetics. 2020;11:585259.
Guo Y, Zeng H, Chang X, Wang C, Cui H. Additional dexamethasone in chemotherapies with carboplatin and paclitaxel could reduce the impaired glycometabolism in rat models. BMC Cancer. 2018;18:81.
Fan Y, Hu D, Li D, Ma C, Tang Y, Tao Q, et al. UCHL3 promotes aerobic glycolysis of pancreatic cancer through upregulating LDHA expression. Clin Transl Oncol. 2021;23:1637–45.
Wang Y, Luo M, Wang F, Tong Y, Li L, Shu Y, et al. AMPK induces degradation of the transcriptional repressor PROX1 impairing branched amino acid metabolism and tumourigenesis. Nat Commun. 2022;13:7215.
Lu H, Shamanna RA, de Freitas JK, Okur M, Khadka P, Kulikowicz T, et al. Cell cycle-dependent phosphorylation regulates RECQL4 pathway choice and ubiquitination in DNA double-strand break repair. Nat Commun. 2017;8:2039.
Khan MA, Tania M. Cordycepin and kinase inhibition in cancer. Drug Discov Today. 2023;28:103481.
Li M, Gao F, Li X, Gan Y, Han S, Yu X, et al. Stabilization of MCL-1 by E3 ligase TRAF4 confers radioresistance. Cell Death Dis. 2022;13:1053.
Zhang Y, Chen S, Peng C. GSK-3β phosphorylation of DHX33 leads to its ubiquitination mediated protein degradation. Cell Signal. 2023;101:110526.
Lee P, Jiang S, Li Y, Yue J, Gou X, Chen SY, et al. Phosphorylation of Pkp1 by RIPK4 regulates epidermal differentiation and skin tumorigenesis. EMBO J. 2017;36:1963–80.
Huang X, McGann JC, Liu BY, Hannoush RN, Lill JR, Pham V, et al. Phosphorylation of Dishevelled by protein kinase RIPK4 regulates Wnt signaling. Science. 2013;339:1441–5.
Pu T, Liu Y, Pei Y, Peng J, Wang Z, Du M, et al. NIR-II fluorescence imaging for the detection and resection of cancerous foci and lymph nodes in early-stage orthotopic and advanced-stage metastatic ovarian cancer models. ACS Appl Mater Interfaces. 2023;15:32226–39.
Lee JH, Park SA, Park IG, Yoon BK, Lee JS, Lee JM. Stem cell properties of gastric cancer stem-like cells under stress conditions are regulated via the c-Fos/UCH-L3/β-catenin axis. Mol Cells. 2023;46:476–85.
Lee JE, Lim YH, Kim JH. UCH-L1 and UCH-L3 regulate the cancer stem cell-like properties through PI3K/Akt signaling pathway in prostate cancer cells. Anim Cells Syst. 2021;25:312–22.
Liu J, Zhang Y, Zeng Q, Zeng H, Liu X, Wu P, et al. Delivery of RIPK4 small interfering RNA for bladder cancer therapy using natural halloysite nanotubes. Sci Adv. 2019;5:eaaw6499.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Number: 81872430), the Fund of the China Postdoctoral Science Foundation (Nos. 2019T120281 and 2019M661304), the Heilongjiang Province Postdoctoral Science Foundation (No. LBH-Z18109), Beijing Kanghua Traditional Chinese Medicine, the Western Medicine Development Foundation (No. KH-2021-LQJJ-008) and the Clinical Key Specialty Fund of Anhui Province (No. 2022sjlczdzk).
Author information
Authors and Affiliations
Contributions
Bairong Xia and Jiming Chen contributed to the project design. Bairong Xia provided financial support. Wulin Shan and Wenju Peng performed the majority of the experiments. Wulin Shan, Wenju Peng, and Yao Chen analyzed the data. Wulin Shan wrote this manuscript. Yuan Tian, Wei Shen, Xu Huang, Xiaoyu Li, and Yingyu Dou assisted with the collection and analysis of data from clinical samples. All authors have read and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study involving human participants and animals was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of USTC. Written informed consent was obtained from all patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shan, W., Peng, W., Chen, Y. et al. GSK3β and UCHL3 govern RIPK4 homeostasis via deubiquitination to enhance tumor metastasis in ovarian cancer. Oncogene (2024). https://doi.org/10.1038/s41388-024-03040-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-024-03040-1