Abstract
Background
This study aimed to explore the functions of ubiquitin-specific protease 5 (USP5) in the endothelial inflammation of Kawasaki disease (KD).
Methods
USP5 expression levels in HCAECs were examined after stimulation with TNFα or KD sera. The inflammatory cytokine expression level and nuclear factor κB (NF-κB) signaling activation proteins were also investigated in HCAECs by using USP5 overexpression/knockdown lentivirus as well as its small molecule inhibitor vialinin A.
Results
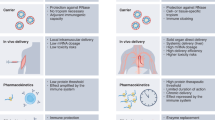
USP5 expression level is upregulated in HCAECs after stimulation with KD sera. Similarly, the USP5 expression level is also increased in a time- and dose-dependent manner upon TNFα stimulation in HCAECs. Moreover, USP5 sustains proinflammatory cytokine production and NF-κB signaling activation, whereas USP5 knockdown causes the proinflammatory cytokine levels to decrease and suppress NF-κB signaling activation. Notably, the USP5 inhibitor vialinin A can suppress the expression of inflammatory genes induced by TNFα and IL-1β in HCAECs.
Conclusions
Our study identified USP5 as a positive regulator of TNFα production and its downstream signaling activation during the inflammatory responses in HCAECs, and demonstrated that its inhibitor vialinin A might serve as a candidate drug for KD therapy to prevent the excessive production of proinflammatory cytokines.
Impact
-
USP5 is upregulated in human coronary artery endothelial cells (HCAECs) whether incubated with acute KD sera or TNFα in vitro.
-
USP5 promotes proinflammatory cytokine expression by sustaining NF-κB signaling activation in HCAECs.
-
The USP5 inhibitor vialinin A can suppress the expression levels of proinflammatory cytokines in HCAEC, thus providing a novel mechanism and intervention strategy in KD therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Cohen, E. & Sundel, R. Kawasaki Disease at 50 Years. JAMA Pediatr. 170, 1093–1099 (2016).
McCrindle, B. W. et al. Diagnosis, treatment, and Long-Term management of kawasaki disease: A scientific statement for health professionals from the american heart association. Circulation. 135, (2017).
Takahashi, K., Oharaseki, T., Yokouchi, Y., Hiruta, N. & Naoe, S. Kawasaki disease as a systemic vasculitis in childhood. Ann. Vasc. Dis. 3, 173–181 (2010).
Sun, L. et al. Changes in profiles of kawasaki disease noted over time in suzhou, china. Cardiology 141, 25–31 (2018).
Noval, R. M. & Arditi, M. Kawasaki disease: Pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 16, 391–405 (2020).
Wang, Y. et al. The role of Ca2+/NFAT in Dysfunction and Inflammation of Human Coronary Endothelial Cells induced by Sera from patients with Kawasaki disease. Sci. Rep.-UK. 10, (2020).
Ueno, K. et al. Disruption of endothelial cell homeostasis plays a key role in the early pathogenesis of coronary artery abnormalities in kawasaki disease. Sci. Rep.-UK. 7, (2017).
Jia, C. et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 10, (2019).
Huang, J., Wu, S., Cao, S., Zhu, X. & Zhang, S. Neutrophil-Derived semaphorin 4D induces inflammatory cytokine production of endothelial cells via different plexin receptors in kawasaki disease. Biomed. Res. Int. 2020, 1–11 (2020).
Ning, F. et al. Structure and function of USP5: Insight into physiological and pathophysiological roles. Pharmacol. Res. 157, 104557 (2020).
Yoshioka, Y. et al. Ubiquitin-specific peptidase 5, a target molecule of vialinin a, is a key molecule of TNF-alpha production in RBL-2H3 cells. PLoS One 8, e80931 (2013).
Kummari, E. et al. Activity-Based proteomic profiling of deubiquitinating enzymes in Salmonella-Infected macrophages leads to identification of putative function of UCH-L5 in inflammasome regulation. PLoS One 10, e0135531 (2015).
Aksentijevich, I. & Zhou, Q. NF-ƙB pathway in autoinflammatory diseases: Dysregulation of protein modifications by ubiquitin defines a new category of autoinflammatory diseases. Front. Immunol. 8, 399 (2017).
Ichiyama, T. et al. NF-κB activation in peripheral blood Monocytes/Macrophages and T cells during acute kawasaki disease. Clin. Immunol. 99, 373–377 (2001).
Onose, J. et al. Vialinin a, a novel potent inhibitor of TNF-alpha production from RBL-2H3 cells. Biol. Pharm. Bull. 31, 831–833 (2008).
Sonowal, H., Shukla, K., Kota, S., Saxena, A. & Ramana, K. V. Vialinin A, an edible Mushroom-Derived p-Terphenyl antioxidant, prevents VEGF-Induced neovascularization in vitro and in vivo. Oxid. Med. Cell. Longev. 2018, 1–10 (2018).
Yamaji, N. et al. TNF-alpha blockers for the treatment of Kawasaki disease in children. Cochrane Database Syst. Rev. 8, CD012448 (2019).
Onose, J. et al. Inhibitory effects of vialinin a and its analog on tumor necrosis factor-α release and production from RBL-2H3 cells. Cell. Immunol. 279, 140–144 (2012).
Qian, G. et al. Ubiquitin specific protease 5 negatively regulates the IFNs-mediated antiviral activity via targeting SMURF1. Int. Immunopharmacol. 87, 106763 (2020).
Zhou, Q. et al. USP15 potentiates NF-ƙB activation by differentially stabilizing TAB2 and TAB3. Febs. J. 287, 3165–3183 (2020).
Xie, C. et al. Vialinin A, a novel 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenger from an edible mushroom in China. Biosci. Biotechnol. Biochem. 69, 2326–2332 (2005).
Radulović, N., Quang, D. N., Hashimoto, T., Nukada, M. & Asakawa, Y. Terrestrins A–G: p-Terphenyl derivatives from the inedible mushroom Thelephora terrestris. Phytochemistry 66, 1052–1059 (2005).
Fujimaru, T. et al. Decreased levels of inflammatory cytokines in immunoglobulin-resistant Kawasaki disease after plasma exchange. Cytokine 70, 156–160 (2014).
Stock, A. T., Jama, H. A., Hansen, J. A. & Wicks, I. P. TNF and IL-1 play essential but temporally distinct roles in driving cardiac inflammation in a murine model of kawasaki disease. J. Immunol. 202, 3151–3160 (2019).
Acknowledgements
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Funding
This project was funded by the National Natural Science Foundation of China (No. 82171797, No. 81971477, No. 81870365, No. 82070512, No. 81970436, No. 81900450), Jiangsu Provincial Social Development Project (SBE2021750252), Jiangsu Provincial Medical Young Talents (QNRC2016756), the Applied Foundational Research of Medical and Health Care of Suzhou City (SYS2019086, SYS2019083, KJXW2018021), Gusu Health Talent Program (GSWS2020038), and Natural Science Foundation for Youth of Wannan Medical College (WK2021F64).
Author information
Authors and Affiliations
Contributions
H.L. and G.Q. conceived the study and drafted the manuscript. C.H., W.W., H.H., J.J., Y.D., X.L., M.H., J.M., and X.P. conducted the experiments, analyzed the data, and participated in the discussion. All authors read and approved the final draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, C., Wang, W., Huang, H. et al. Kawasaki disease: ubiquitin-specific protease 5 promotes endothelial inflammation via TNFα-mediated signaling. Pediatr Res 93, 1883–1890 (2023). https://doi.org/10.1038/s41390-022-02341-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02341-z