Abstract
Background
Lyme disease is common among children and adolescents. Antibiotic treatment is effective, yet some patients report persistent symptoms following treatment, with or without functional impairment. This study characterized long-term outcome of pediatric patients with Lyme disease and evaluated the case definition of post-treatment Lyme disease (PTLD) syndrome.
Methods
The sample included 102 children with confirmed Lyme disease diagnosed 6 months—10 years prior to enrollment (M = 2.0 years). Lyme diagnosis and treatment information was extracted from the electronic health record; parent report identified presence, duration, and impact of symptoms after treatment. Participants completed validated questionnaires assessing health-related quality of life, physical mobility, fatigue, pain, and cognitive impact.
Results
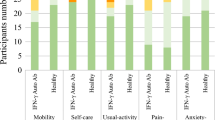
Most parents reported their child’s symptoms resolved completely, although time to full resolution varied. Twenty-two parents (22%) indicated their child had at least one persistent symptom >6 months post-treatment, 13 without functional impairment (PTLD symptoms) and 9 with functional impairment (PTLD syndrome). Children with PTLD syndrome had lower parent-reported Physical Summary scores and greater likelihood of elevated fatigue.
Conclusions
In the current study, most children with Lyme disease experienced full resolution of symptoms, including those who initially met PTLD syndrome criteria. Effective communication about recovery rates and common symptoms that may persist post-treatment is needed.
Impact
-
The majority of pediatric patients treated for all stages of Lyme disease reported full resolution of symptoms within 6 months.
-
22% of pediatric patients reported one or more symptom persisting >6 months, 9% with and 13% without accompanying functional impairment.
-
Effective communication with families about recovery rates and common symptoms that may persist post-treatment of Lyme disease is needed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data collected for the purposes of this study and analyzed during the current study are available from the corresponding author on reasonable request.
References
Marques, A. R., Strle, F. & Wormser, G. P. Comparison of Lyme disease in the United States and Europe. Emerg. Infect. Dis. 27, 2017–2024 (2021).
Schwartz, A. M., Kugeler, K. J., Nelson, C. A., Marx, G. E. & Hinckley, A. F. Use of commercial claims data for evaluating trends in Lyme disease diagnoses, United States, 2010–2018. Emerg. Infect. Dis. 27, 499–507 (2021).
Kugeler, K. J., Schwartz, A. M., Delorey, M. J., Mead, P. S. & Hinckley, A. F. Estimating the frequency of Lyme disease diagnoses, United States, 2010–2018. Emerg. Infect. Dis. 27, 616–619 (2021).
Kugeler, K. J., Mead, P. S., Schwartz, A. M. & Hinckley, A. F. Changing trends in age and sex distributions of Lyme disease-United States, 1992–2016. Public Health Rep. 137, 655–659 (2022).
Lantos, P. M. et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis and Treatment of Lyme Disease. Clin. Infect. Dis. 72, 1–8 (2021).
Turk, S. P. et al. Post-treatment Lyme disease symptoms score: developing a new tool for research. PLoS One 14, e0225012 (2019).
Wormser, G. P. et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 43, 1089–1134 (2006).
Aucott, J. N., Crowder, L. A. & Kortte, K. B. Development of a foundation for a case definition of post-treatment Lyme disease syndrome. Int J. Infect. Dis. 17, e443–e449 (2013).
Aucott, J. N. Posttreatment Lyme disease syndrome. Infect. Dis. Clin. North Am. 29, 309–323 (2015).
Schmid, H. & Heininger, U. Post-treatment Lyme disease syndrome-what it might be and what it is not. Pediatr. Infect. Dis. J. 40, S31–S34 (2021).
Marques, A., Lemieux, J. & Hu, L. The widening gyre: controversies in Lyme disease. in Lyme Disease and Relapsing Fever Spirochetes: Genomics, Molecular Biology, Host Interactions and Disease Pathogenesis (eds. J.D. Radolf & D.S. Samuels) 685–702, Caister Academic Press, UK (2021).
McCarthy, C. A., Helis, J. A. & Daikh, B. E. Lyme disease in children. Infect. Dis. Clin. North Am. 36, 593–603 (2022).
Nowakowski, J. et al. Long-term follow-up of patients with culture-confirmed Lyme disease. Am. J. Med. 115, 91–96 (2003).
Weitzner, E. et al. Long-term assessment of post-treatment symptoms in patients with culture-confirmed early Lyme disease. Clin. Infect. Dis. 61, 1800–1806 (2015).
Borsic, K., Blagus, R., Cerar, T., Strle, F. & Stupica, D. Clinical course, serologic response, and long-term outcome in elderly patients with early Lyme borreliosis. J. Clin. Med. 7, 506 (2018).
Hirsch, A. G. et al. Risk factors and outcomes of treatment delays in Lyme disease: a population-based retrospective cohort study. Front. Med. (Lausanne) 7, 560018 (2020).
Wormser, G. P. et al. Evaluation of selected variables to determine if any had predictive value for, or correlated with, residual symptoms at approximately 12 months after diagnosis and treatment of early Lyme disease. Diagn. Microbiol. Infect. Dis. 100, 115348 (2021).
Wills, A. B. et al. Long-term follow-up of patients with Lyme disease: longitudinal analysis of clinical and quality-of-life measures. Clin. Infect. Dis. 62, 1546–1551 (2016).
Aucott, J. N. et al. Risk of post-treatment Lyme disease in patients with ideally-treated early Lyme disease: a prospective cohort study. Int J. Infect. Dis. 116, 230–237 (2022).
Chason, M. E., Monaghan, M., Wang, J., Cheng, Y. & DeBiasi, R. L. Symptom resolution in pediatric patients with Lyme disease. J. Pediatr. Infect. Dis. Soc. 8, 170–173 (2019).
Sood, S. K. Lyme disease in children. Infect. Dis. Clin. North Am. 29, 281–294 (2015).
Mac, S. et al. Long-term sequelae and health-related quality of life associated with Lyme disease: a systematic review. Clin. Infect. Dis. 71, 440–452 (2020).
Tory, H. O., Zurakowski, D. & Sundel, R. P. Outcomes of children treated for Lyme arthritis: results of a large pediatric cohort. J. Rheumatol. 37, 1049–1055 (2010).
Forde, K. M. et al. The clinical presentation, treatment and outcome of serologically confirmed paediatric Lyme disease in the Republic of Ireland over a 5-year period: a retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 40, 725–734 (2021).
Gerber, M. A., Shapiro, E. D., Burke, G. S., Parcells, V. J. & Bell, G. L. Lyme disease in children in southeastern Connecticut. Pediatric Lyme Disease Study Group. N. Engl. J. Med. 335, 1270–1274 (1996).
Skogman, B. H. et al. Long-term clinical outcome after Lyme neuroborreliosis in childhood. Pediatrics 130, 262–269 (2012).
Nesgos, A. T., Harrington, L. C. & Mader, E. M. Experience and knowledge of Lyme disease: a scoping review of patient-provider communication. Ticks Tick. Borne Dis. 12, 101714 (2021).
Centers for Disease Control and Prevention. Lyme Disease (Borrelia burgdorferi) 2017 Case Definition 2017 [updated 04/16/2021]. Available from: https://ndc.services.cdc.gov/case-definitions/lyme-disease-2017/.
Landgraf, J., Abetz, L. & Ware, J. The CHQ user’s manual. Boston: The Health Institute, New England Medical Center, 235–239 (1996).
Wrotniak, B. H., Schall, J. I., Brault, M. E., Balmer, D. F. & Stallings, V. A. Health-related quality of life in children with sickle cell disease using the Child Health Questionnaire. J. Pediatr. Health Care 28, 14–22 (2014).
Waters, E., Davis, E., Nicolas, C., Wake, M. & Lo, S. K. The impact of childhood conditions and concurrent morbidities on child health and well-being. Child Care Health Dev. 34, 418–429 (2008).
Varni, J. W. et al. Psychometric properties of the PROMIS (R) pediatric scales: precision, stability, and comparison of different scoring and administration options. Qual. Life Res. 23, 1233–1243 (2014).
Varni, J. W. et al. PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. J. Pain. 11, 1109–1119 (2010).
Mann, C. M. et al. Identifying clinically meaningful severity categories for PROMIS pediatric measures of anxiety, mobility, fatigue, and depressive symptoms in juvenile idiopathic arthritis and childhood-onset systemic lupus erythematosus. Qual. Life Res. 29, 2573–2584 (2020).
Irwin, D. E. et al. Development of six PROMIS pediatrics proxy-report item banks. Health Qual. Life Outcomes 10, 22 (2012).
Penny, A. M., Waschbusch, D. A., Klein, R. M., Corkum, P. & Eskes, G. Developing a measure of sluggish cognitive tempo for children: Content validity, factor structure, and reliability. Psychol. Assess. 21, 380–389 (2009).
Smith, Z. R. et al. Evaluating the structure of sluggish cognitive tempo using confirmatory factor analytic and bifactor modeling with parent and youth ratings. Assessment 25, 99–111 (2018).
Bechtold, K. T., Rebman, A. W., Crowder, L. A., Johnson-Greene, D. & Aucott, J. N. Standardized symptom measurement of individuals with early Lyme disease over time. Arch. Clin. Neuropsychol. 32, 129–141 (2017).
Eikeland, R. et al. Patient-reported outcome after treatment for definite Lyme neuroborreliosis. Brain Behav. 10, e01595 (2020).
Dersch, R., Sommer, H., Rauer, S. & Meerpohl, J. J. Prevalence and spectrum of residual symptoms in Lyme neuroborreliosis after pharmacological treatment: a systematic review. J. Neurol. 263, 17–24 (2016).
Funding
This work was supported by Clinical and Translational Science Institute at Children’s National (CTSI-CN) [Grant number UL1TR001876-05 and UL1TR001876-02S1] (R.D., M.M., J.B., M.G., S.N.) and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (A.M.).
Author information
Authors and Affiliations
Contributions
M.M. and R.D. made substantial contributions to the conception and design of the study, contributed to the analysis and interpretation of data, drafted the article, and revised it critically for important intellectual content. A.M. made substantial contributions to the conception and design of the study, contributed to the analysis and interpretation of data, drafted the article, and revised the article critically for important intellectual content. S.N. made substantial contributions to the acquisition of data and analysis of data, drafted the article, and critically reviewed the manuscript. M.G. and J.B. made substantial contributions to the analysis and interpretation of data and revised the article critically for important intellectual content. All authors provided final approval of the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
M.M. is currently employed by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. All data for this manuscript was collected prior to her employment at the National Institutes of Health. A.M. is employed by the National Institute of Allergy and Infectious Diseases, National Institutes of Health; A.M. has a patent (US 8,926,989) and serves as an unpaid scientific advisor to the Global Lyme Alliance and the American Lyme Disease Foundation. R.L.D., S.N., M.G., and J.B. declare no conflicts of interest.
Ethics approval and consent to participate
All participating parents and adult patients aged 18 years and up provided consent to participate in the study; adolescents ages 10 and up provided assent to participate in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Monaghan, M., Norman, S., Gierdalski, M. et al. Pediatric Lyme disease: systematic assessment of post-treatment symptoms and quality of life. Pediatr Res 95, 174–181 (2024). https://doi.org/10.1038/s41390-023-02577-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02577-3
This article is cited by
-
Reply to Correspondence
Pediatric Research (2024)
-
Correspondence re: M Monaghan et al. Pediatric Lyme disease: systematic assessment of post-treatment symptoms and quality of life
Pediatric Research (2024)