Abstract
Background
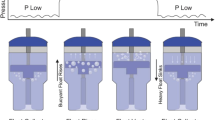
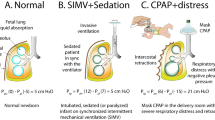
Atelectasis is a common complication in neonatal anesthesia. Lung ultrasound (LUS) can be used intraoperatively to evaluate and recognize atelectatic lung areas. Hypotheses for the study are: (1) The use of LUS to guide choice of best positive end-expiratory pressure (PEEP) can lead to reduction of FiO2 to achieve same saturations of oxygen (SpO2). (2) In a less de-recruited lung, there will be less postoperative pulmonary complications. (3) Static respiratory system compliance could be different. (4) Hemodynamic parameters and amount of fluids infused or need for vasopressors intraoperatively could be different.
Methods
We propose a randomized controlled trial that compares standard PEEP settings with LUS-guided PEEP choice in patients under 2 months of age undergoing general anesthesia.
Results
The primary aim is to determine whether LUS-guided PEEP choice in neonatal anesthesia, compared to standard PEEP choice, can lead to reduction of FiO2 applied to the ventilatory setting in order to maintain same SpO2s. Secondary aims are to determine whether patients treated with LUS-guided PEEP will develop less postoperative pulmonary complications, will have a significant difference in hemodynamic parameters and amount of fluids or vasopressors infused, and in static respiratory system compliance.
Conclusions
We expect a significant reduction of FiO2 in LUS-guided ventilation.
Impact
-
Lung atelectasis is extremely common in neonatal anesthesia, because of the physiology of the neonatal lung and chest wall and leads to hypoxemia, being a lung area with a perfusion/ventilation mismatch.
-
Raising inspired fraction of oxygen can overcome temporarily hypoxemia but oxygen is a toxic compound for newborns. Lung ultrasound (LUS) can detect atelectasis at bedside and be used to optimize ventilator settings including choice of positive end-expiratory pressure (PEEP).
-
This randomized controlled trial (RCT) aims at demonstrating that LUS-guided choice of best PEEP during neonatal anesthesia can lead to reduction of inspired fractions of oxygen to keep same peripheral saturations SpO2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
After study completion, data will be available upon reasonable request.
References
Bruins, S., Sommerfield, D., Powers, N. & von Ungern‐Sternberg, B. S. Atelectasis and lung recruitment in pediatric anesthesia: an educational review. Pediatr. Anesth. 32, 321–329 (2022).
Zeng, C., Lagier, D., Lee, J. W. & Vidal Melo, M. F. Perioperative pulmonary atelectasis: Part I. Biology and mechanisms. Anesthesiology 136, 181–205 (2022).
de Graaff, J. C. et al. Incidence of intraoperative hypoxemia in children in relation to age. Anesth. Analg. 117, 169–175 (2013).
Lai-Fook, S. J. & Rodarte, J. R. Pleural pressure distribution and its relationship to lung volume and interstitial pressure. J. Appl. Physiol. 70, 967–978 (1991).
Warner, D. O., Warner, M. A. & Ritman, E. L. Atelectasis and chest wall shape during halothane anesthesia. Anesthesiology 85, 49–59 (1996).
Joyce, C. J. & Williams, A. B. Kinetics of absorption atelectasis during anesthesia: a mathematical model. J. Appl. Physiol. 86, 1116–1125 (1999).
Hedenstierna, G. & Rothen, H. U. in Comprehensive Physiology 1st edn (ed. Terjung, R.) 69–96 (Wiley, 2012).
Edmark, L., Auner, U., Enlund, M., Östberg, E. & Hedenstierna, G. Oxygen concentration and characteristics of progressive atelectasis formation during anaesthesia: oxygen and dynamics of atelectasis formation during anaesthesia. Acta Anaesthesiol. Scand. 55, 75–81 (2011).
Magnusson, L. & Spahn, D. R. New concepts of atelectasis during general anaesthesia. Br. J. Anaesth. 91, 61–72 (2003).
Lutterbey, G. et al. Atelectasis in children undergoing either propofol infusion or positive pressure ventilation anesthesia for magnetic resonance imaging. Pediatr. Anesth. 17, 121–125 (2007).
Schumann, S., Feth, A., Borgmann, S. & Wirth, S. Dependency of respiratory system mechanics on positive end‐expiratory pressure and recruitment maneuvers in lung healthy pediatric patients—a randomized crossover study. Pediatr. Anaesth. 30, 905–911 (2020).
Laver, M. B., Morgan, J., Bendixen, H. H. & Radford, E. P. Lung volume, compliance, and arterial oxygen tensions during controlled ventilation. J. Appl. Physiol. 19, 725–733 (1964).
Nunn, J. F., Bergman, N. A. & Coleman, A. J. Factors influencing the arterial oxygen tension during anaesthesia with artificial ventilation. Br. J. Anaesth. 37, 898–914 (1965).
Frank, L., Bucher, J. R. & Roberts R. J. Oxygen toxicity in neonatal and adult animals of various species. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 45, 699–704 (1978).
Kulkarni, A. C., Kuppusamy, P. & Parinandi, N. Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid. Redox Signal. 9, 1717–1730 (2007).
Saugstad, O. D. Bronchopulmonary dysplasia—oxidative stress and antioxidants. Semin. Neonatol. 8, 39–49 (2003).
Tipple, T. E. & Ambalavanan, N. Oxygen toxicity in the neonate. Clin. Perinatol. 46, 435–447 (2019).
Maltepe, E. & Saugstad, O. D. Oxygen in health and disease: regulation of oxygen homeostasis-clinical implications. Pediatr. Res. 65, 261–268 (2009).
Saugstad, O. D., Sejersted, Y., Solberg, R., Wollen, E. J. & Bjørås, M. Oxygenation of the newborn: a molecular approach. Neonatology 101, 315–325 (2012).
Short, J. A. & Van Der Walt, J. H. Oxygen in neonatal and infant anesthesia – current practice in the UK. Pediatr. Anesth. 18, 378–387 (2008).
Cereda, M. et al. Positive end-expiratory pressure increments during anesthesia in normal lung result in hysteresis and greater numbers of smaller aerated airspaces. Anesthesiology 119, 1402–1409 (2013).
Biasucci, D. G. et al. Ultrasound-assessed lung aeration correlates with respiratory system compliance in adults and neonates with acute hypoxemic restrictive respiratory failure: an observational prospective study. Respir. Res. 23, 360 (2022).
Lichtenstein, D. A. & Mezière, G. A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 134, 117–125 (2008).
Acosta, C. M. et al. Accuracy of transthoracic lung ultrasound for diagnosing anesthesia-induced atelectasis in children. Anesthesiology 120, 1370–1379 (2014).
Singh, Y. et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit. Care 24, 65 (2020).
Buonsenso, D. et al. Lung ultrasound pattern in healthy infants during the first 6 months of life. J. Ultrasound Med. 39, 2379–2388 (2020).
International Liaison Committee on Lung Ultrasound (ILC-LUS) for the International Consensus Conference on Lung Ultrasound (ICC-LUS), Volpicelli, G. & Elbarbary, M. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 38, 577–591 (2012).
Herring, M. J., Putney, L. F., Wyatt, G., Finkbeiner, W. E. & Hyde, D. M. Growth of alveoli during postnatal development in humans based on stereological estimation. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L338–L344 (2014).
Rice, T. W. et al. Comparison of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or ARDS. Chest 132, 410–417 (2007).
Haines, K. L. & Agarwal, S. Postoperative pulmonary complications—a multifactorial outcome. JAMA Surg. 152, 166 (2017).
Ray, S. et al. PaO2/FiO2 ratio derived from the SpO2/FiO2 ratio to improve mortality prediction using the Pediatric Index of Mortality-3 Score in transported intensive care admissions. Pediatr. Crit. Care Med. 18, e131–e136 (2017).
Li, L. W. et al. Influence of laparoscopic carbon dioxide pneumoperitoneum on neonate circulation and respiration. J. Int. Med. Res. 41, 889–894 (2013).
Funding
This study is unfunded.
Author information
Authors and Affiliations
Contributions
A.C. and U.M.P. designed the study; A.C. and A.G. designed the statistical plan and the power analysis for the study; D.B. coordinated and supervised all aspects of the study, helped to draft the initial manuscript, and reviewed and revised the manuscript. A.C., U.M.P. G. Paladini, D.B., and G. Pelizzo reviewed and revised the protocol and critically reviewed the manuscript for important intellectual content. All authors reviewed the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Camporesi, A., Pierucci, U.M., Paladini, G. et al. Lung ultrasound-guided best positive end-expiratory pressure in neonatal anesthesia: a proposed randomized, controlled study. Pediatr Res 95, 393–396 (2024). https://doi.org/10.1038/s41390-023-02730-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02730-y