Abstract
Background
We investigated whether combining lung ultrasound scores (LUSs) and respiratory system reactance (Xrs) measured by respiratory oscillometry explains the severity of lung disease better than individual parameters alone.
Methods
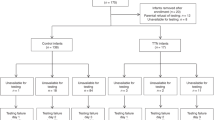
We performed a prospective observational study in very preterm infants. Forced oscillations (10 Hz) were applied using a neonatal mechanical ventilator (Fabian HFOi, Vyaire). We used the simultaneous respiratory severity score (RSS = mean airway pressure × FIO2) as a primary outcome. We built linear mixed-effect models to assess the relationship between Xrs z-score, LUS and RSS and compared nested models using the likelihood ratio test (LRT).
Results
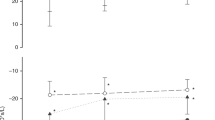
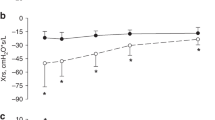
We enrolled 61 infants (median (Q1, Q3) gestational age = 30.00 (26.86, 31.00) weeks) and performed 243 measurements at a postnatal age of 26 (13, 41) days and postmenstrual age of 33.14 (30.46, 35.86) weeks. Xrs z-score and LUS were independently associated with simultaneous RSS (p < 0.001 for both). The model including Xrs and LUS explained the RSS significantly better than Xrs (p value LRT < 0.001) or LUS alone (p value LRT < 0.001).
Conclusions
Combining LUS and Xrs z-score explains the severity of lung disease better than each parameter alone and has the potential to improve the understanding of the underlying pathophysiology.
Impact
-
Combining respiratory system reactance by oscillometry and lung ultrasound score explains the respiratory support requirement (e.g., proxy of the severity of lung disease) significantly better than each parameter alone.
-
We assessed the relationship between lung ultrasound and respiratory system reactance in very preterm infants for the first time.
-
Combining respiratory oscillometry and lung ultrasound has the potential to improve the understanding of respiratory pathophysiology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Laughon, M. et al. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics 123, 1124–1131 (2009).
Collaco, J. M. & McGrath-Morrow, S. A. Respiratory phenotypes for preterm infants, children, and adults: bronchopulmonary dysplasia and more. Ann. Am. Thorac. Soc. 15, 530–538 (2018).
Brat, R. et al. Lung ultrasonography score to evaluate oxygenation and surfactant need in neonates treated with continuous positive airway pressure. JAMA Pediatr. 169, e151797 (2015).
Raimondi, F. et al. Use of neonatal chest ultrasound to predict noninvasive ventilation failure. Pediatrics 134, e1089–e1094 (2014).
Raimondi, F. et al. Lung ultrasound for diagnosing pneumothorax in the critically ill neonate. J. Pediatr. 175, 74–78.e1 (2015).
Raimondi, F. et al. Point-of-care lung ultrasound in neonatology: classification into descriptive and functional applications. Pediatr. Res. 90, 524 (2021).
Raimondi, F. et al. A multicenter lung ultrasound study on transient tachypnea of the neonate. Neonatology 115, 263–268 (2019).
Piastra, M. et al. Lung ultrasound findings in meconium aspiration syndrome. Early Hum. Dev. 90, S41–S43 (2014).
Loi, B. et al. Lung ultrasound to monitor extremely preterm infants and predict bronchopulmonary dysplasia a multicenter longitudinal cohort study. Am. J. Respir. Crit. Care Med. 203, 1398–1409 (2021).
Zannin, E., Neumann, R. P., Dellacà, R. & Schulzke, S. M. Forced oscillation measurements in the first week of life and pulmonary outcome in very preterm infants on noninvasive respiratory support. Pediatr. Res. 86, 382–388 (2019).
Veneroni, C., Wallström, L., Sindelar, R. & Dellacaʼ, R. L. Oscillatory respiratory mechanics on the first day of life improves prediction of respiratory outcomes in extremely preterm newborns. Pediatr. Res. 85, 312–317 (2018).
Zannin, E. et al. Oscillatory mechanics at 36 weeks post-menstrual age as markers of lung disease in preterm infants: a cohort study. European Respiratory J. 59, 2103023 (2022).
Dellacà, R. L. et al. Relationship between respiratory impedance and positive end-expiratory pressure in mechanically ventilated neonates. Intensive Care Med. 39, 511–519 (2013).
Mayo, P. H. et al. Thoracic ultrasonography: a narrative review. Intensive Care Med. 45, 1200–1211 (2019).
Chiumello, D. et al. Assessment of lung aeration and recruitment by CT scan and ultrasound in acute respiratory distress syndrome patients. Crit. Care Med. 46, 1761–1768 (2018).
Bouhemad, B. et al. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am. J. Respir. Crit. Care Med. 183, 341–347 (2011).
Soldati, G. et al. On the physical basis of pulmonary sonographic interstitial syndrome. J. Ultrasound Med. 35, 2075–2086 (2016).
Dellacà, R. L. et al. Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur. Respir. J. 23, 232–240 (2004).
Dellaca, R. L. et al. Lung recruitment assessed by total respiratory system input reactance. Intensive Care Med. 35, 2164–2172 (2009).
Dellaca, R. L. et al. Optimisation of positive end-expiratory pressure by forced oscillation technique in a lavage model of acute lung injury. Intensive Care Med. 37, 1021–1030 (2011).
Bates, J. H., Schmalisch, G., Filbrun, D. & Stocks, J. Tidal breath analysis for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur. Respir. J. 16, 1180–1192 (2000).
Pigmans, R. R. W. P. et al. Influence of neonatal endotracheal tube dimensions on oscillometry-acquired reactance: a bench study. Physiol. Meas. 44 (2023).
Zannin, E. et al. Oscillatory mechanics in very preterm infants on continuous positive airway pressure support: reference values. Pediatr. Pulmonol. 58, 746–752 (2023).
Lavizzari, A. et al. Respiratory mechanics during NCPAP and HHHFNC at equal distending pressures. Arch. Dis. Child Fetal Neonatal Ed. 99, F315–F320 (2014).
Cohen, J., Cohen, P., West, S. G., Aiken, L.S. (eds) Applied multiple regression/correlation analysis for the behavioral sciences. Google Libri. 3rd edn (Lawrence Erlbaum Associates, Publishers, Mahwah, New Jersey, 2002).
Biasucci, D. G. et al. Ultrasound-assessed lung aeration correlates with respiratory system compliance in adults and neonates with acute hypoxemic restrictive respiratory failure: an observational prospective study. Respir. Res. 23, 360 (2022).
Ntoumenopoulos, G., Buscher, H. & Scott, S. Lung ultrasound score as an indicator of dynamic lung compliance during veno-venous extra-corporeal membrane oxygenation. Int. J. Artif. Organs 44, 194–198 (2021).
Loi, B. et al. Lung ultrasound features and relationships with respiratory mechanics of evolving BPD in preterm rabbits and human neonates. J. Appl. Physiol. 131, 895–904 (2021).
Connors, J. & Gibbs, K. Bronchopulmonary dysplasia: a multidisciplinary approach to management. Curr. Pediatr. Rep. 7, 83–89 (2019).
Picano, E. & Pellikka, P. A. Ultrasound of extravascular lung water: a new standard for pulmonary congestion. Eur. Heart J. 37, 2097 (2016).
Sett, A. et al. Lung ultrasound of the dependent lung detects real-time changes in lung volume in the preterm lamb. Arch. Dis. Child Fetal Neonatal Ed. 108, 51 (2023).
Sett, A. et al. Quantitative lung ultrasound detects dynamic changes in lung recruitment in the preterm lamb. Pediatr. Res. 93, 1591–1598 (2023).
Pryor, E. J. et al. Quantifying lung aeration in neonatal lambs at birth using lung ultrasound. Front. Pediatr. 10, 990923 (2022).
Sett, A. et al. Estimating preterm lung volume: a comparison of lung ultrasound, chest radiography, and oxygenation. J. Pediatr. 259, 113437 (2023).
Cattarossi, L., Copetti, R., Poskurica, B. & Miserocchi, G. Surfactant administration for neonatal respiratory distress does not improve lung interstitial fluid clearance: echographic and experimental evidence. J. Perinat. Med. 38, 557–563 (2010).
Dellacà, R. L. et al. Changes in the mechanical properties of the respiratory system during the development of interstitial lung edema. Respir. Res. 9, 1–9 (2008).
Shepherd, E. G. et al. Infant pulmonary function testing and phenotypes in severe bronchopulmonary dysplasia. Pediatrics 141, e20173350 (2018).
Sehgal, A. et al. A new look at bronchopulmonary dysplasia: postcapillary pathophysiology and cardiac dysfunction. Pulm. Circ. 6, 508–515 (2016).
Sehgal, A. et al. The left heart, systemic circulation, and bronchopulmonary dysplasia: relevance to pathophysiology and therapeutics. J. Pediatr. 225, 13–22.e2 (2020).
Pierro, M. et al. Endotypes of prematurity and phenotypes of bronchopulmonary dysplasia: toward personalized neonatology. J. Pers. Med. 12, 687 (2022).
Hall, G. L., Hantos, Z., Sly, P. D. & Wildhaber, J. H. Contribution of nasal pathways to low frequency respiratory impedance in infants. Thorax 57, 396 (2002).
Veneroni, C., Wallström, L., Sindelar, R. & Dellacaʼ, R. L. Oscillatory respiratory mechanics on the first day of life improves prediction of respiratory outcomes in extremely preterm newborns. Pediatr. Res. 85, 312–317 (2019).
Author information
Authors and Affiliations
Contributions
C.R. contributed to the study design, performed lung ultrasound scans and scored the images, interpreted the results and approved the final manuscript as submitted. E.Z. contributed to the study design, processed the respiratory oscillometry data, performed formal analysis and drafted the first version of the manuscript. R.L.D. interpreted the data, revised the manuscript and approved the final manuscript as submitted. M.L.V. interpreted the data, critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
R.L.D. reports that Politecnico di Milano received research grants from Vyaire and licensed a patent for using FOT to assess lung volume recruitment to Vyaire. C.R. has received honoraria for lectures from Vyaire. The other authors have nothing to disclose. None of the authors received any form of payment to produce the manuscript.
Ethics approval and consent to participate
The local ethics committee (nr. 3804/21) approved the study and written informed consent was obtained from parents before enrollment.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rigotti, C., Zannin, E., Dellacà, R.L. et al. Combining lung ultrasound and oscillatory mechanics for assessing lung disease in very preterm infants. Pediatr Res 95, 1022–1027 (2024). https://doi.org/10.1038/s41390-023-02829-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02829-2
This article is cited by
-
The best of both worlds: Refining respiratory phenotypes through combined non-invasive lung monitoring
Pediatric Research (2023)