Abstract
Objective
Neonates born with fetal inflammatory response (FIR) are at increased risk for adverse neonatal outcomes. Our objective was to determine whether FIR and its severity is associated with severity of necrotizing enterocolitis (NEC) in preterm infants.
Methods
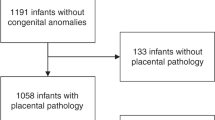
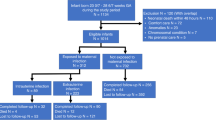
A case-control retrospective study of infants <33 weeks gestational age or <1500 g birthweight, including 260 with stage I–III NEC and 520 controls matched for gestational age. Placental pathology was evaluated, and FIR progression and its severity were defined according to Amsterdam classification.
Results
In this study, mild FIR (i.e., stage 1 FIR) was present in 52 controls (10.0%) and 22 infants with stage I–III NEC (8.5%), while moderate to severe FIR (i.e., ≥stage 2 FIR) was present in 16 controls (3.1%) and 47 infants with stage I–III NEC (18.1%). Both stage and grade of FIR were associated with stage of NEC (P < 0.001). On multinomial logistic regression, stage III NEC was associated with stage of FIR (P < 0.001).
Conclusion
This is the first report demonstrating the association between progression and increasing severity of FIR and stage of NEC.
Impact
-
Fetal Inflammatory Response (FIR) and its progression and severity are associated with the stages of necrotizing enterocolitis (NEC).
-
This is the first study demonstrating the impact of progression and severity of FIR on stage III NEC.
-
These observations provide additional insight into understanding the impact of intrauterine exposure to inflammation on the severity of NEC in preterm infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 314, 1039–1051 (2015).
Papillon, S., Castle, S. L., Gayer, C. P. & Ford, H. R. Necrotizing enterocolitis: contemporary management and outcomes. Adv. Pediatr. 60, 263–279 (2013).
Stey, A. et al. Outcomes and costs of surgical treatments of necrotizing enterocolitis. Pediatrics 135, e1190–e1197 (2015).
Zhang, Y. et al. Necrotizing enterocolitis requiring surgery: outcomes by intestinal location of disease in 4371 infants. J. Pediatr. Surg. 46, 1475–1481 (2011).
Fitzgibbons, S. C. et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg. 44, 1072–1075 (2009). discussion 1075-1076.
Robinson, J. R. et al. Surgical necrotizing enterocolitis. Semin Perinatol. 41, 70–79 (2017).
Hintz, S. R. et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 115, 696–703 (2005).
Garzoni, L., Faure, C. & Frasch, M. G. Fetal cholinergic anti-inflammatory pathway and necrotizing enterocolitis: the brain-gut connection begins in utero. Front. Integr. Neurosci. 7, 57 (2013).
Bisquera, J. A., Cooper, T. R. & Berseth, C. L. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics 109, 423–428 (2002).
Lin, P. W. & Stoll, B. J. Necrotising enterocolitis. Lancet 368, 1271–1283 (2006).
Wendel, G. D. Jr. et al. Identification of Treponema pallidum in amniotic fluid and fetal blood from pregnancies complicated by congenital syphilis. Obstet. Gynecol. 78, 890–895 (1991).
Morgan-Capner, P., Rodeck, C. H., Nicolaides, K. H. & Cradock-Watson, J. E. Prenatal detection of rubella-specific IgM in fetal sera. Prenat. Diagn. 5, 21–26 (1985).
Thilaganathan, B., Carroll, S. G., Plachouras, N., Makrydimas, G. & Nicolaides, K. H. Fetal immunological and haematological changes in intrauterine infection. Br. J. Obstet. Gynaecol. 101, 418–421 (1994).
Donders, G. G., Moerman, P., Caudron, J. & Van Assche, F. A. Intra-uterine Candida infection: a report of four infected fetusses from two mothers. Eur. J. Obstet. Gynecol. Reprod. Biol. 38, 233–238 (1991).
Carroll, S. G. & Nicolaides, K. H. Fetal haematological response to intra-uterine infection in preterm prelabour amniorrhexis. Fetal Diagn. Ther. 10, 279–285 (1995).
Abrams, E. T. et al. Malaria during pregnancy and foetal haematological status in Blantyre, Malawi. Malar. J. 4, 39 (2005).
Gomez, R. et al. The fetal inflammatory response syndrome. Am. J. Obstet. Gynecol. 179, 194–202 (1998).
Yoon, B. H. et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am. J. Obstet. Gynecol. 183, 1124–1129 (2000).
Kim, C. J., Yoon, B. H., Park, S. S., Kim, M. H. & Chi, J. G. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum. Pathol. 32, 623–629 (2001).
Pacora, P. et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J. Matern. Fetal Neonatal Med. 11, 18–25 (2002).
Oh, J. W., Park, C. W., Moon, K. C., Park, J. S. & Jun, J. K. The relationship among the progression of inflammation in umbilical cord, fetal inflammatory response, early-onset neonatal sepsis, and chorioamnionitis. PLoS One 14, e0225328 (2019).
Been, J. V. et al. Histologic chorioamnionitis, fetal involvement, and antenatal steroids: effects on neonatal outcome in preterm infants. Am. J. Obstet. Gynecol. 201, 587 (2009). e581-588.
Elimian, A. et al. Histologic chorioamnionitis, antenatal steroids, and perinatal outcomes. Obstet. Gynecol. 96, 333–336 (2000).
Lau, J. et al. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am. J. Obstet. Gynecol. 193, 708–713 (2005).
Goldenberg, R. L. et al. The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection. Am. J. Obstet. Gynecol. 195, 1020–1024 (2006).
Ogunyemi, D., Murillo, M., Jackson, U., Hunter, N. & Alperson, B. The relationship between placental histopathology findings and perinatal outcome in preterm infants. J. Matern. Fetal Neonatal. Med. 13, 102–109 (2003).
Been, J. V., Lievense, S., Zimmermann, L. J., Kramer, B. W. & Wolfs, T. G. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J. Pediatr. 162, 236–242 e232 (2013).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Andrews, R. E. & Coe, K. L. Clinical presentation and multifactorial pathogenesis of necrotizing enterocolitis in the preterm infant. Adv. Neonatal Care 21, 349–355 (2021).
Duric, B. et al. Effect of time of diagnosis to surgery on outcome, including long-term neurodevelopmental outcome, in necrotizing enterocolitis. Pediatr. Surg. Int. 39, 2 (2022).
Greer, L. G. et al. An immunologic basis for placental insufficiency in fetal growth restriction. Am. J. Perinatol. 29, 533–538 (2012).
Mir, I. N. et al. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am. J. Obstet. Gynecol. 213, 849 (2015). e841-847.
Redline, R. W. & Abramowsky, C. R. Clinical and pathologic aspects of recurrent placental villitis. Hum. Pathol. 16, 727–731 (1985).
Redline, R. W., Heller, D., Keating, S. & Kingdom, J. Placental diagnostic criteria and clinical correlation-a workshop report. Placenta 26, S114–S117 (2005).
Khong, T. Y. et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch. Pathol. Lab Med 140, 698–713 (2016).
Olsen, I. E., Groveman, S. A., Lawson, M. L., Clark, R. H. & Zemel, B. S. New intrauterine growth curves based on United States data. Pediatrics 125, e214–e224 (2010).
Olsen, I. E. et al. BMI curves for preterm infants. Pediatrics 135, e572–581, (2015).
Andrews, W. W. et al. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am. J. Obstet. Gynecol. 195, 803–808 (2006).
Garg, P. M. et al. Association of placental pathologic findings with the severity of necrotizing enterocolitis in preterm infants—a matched case-control study. Fetal Pediatr. Pathol. 42, 187–197 (2023).
Neu, J., Mshvildadze, M. & Mai, V. A roadmap for understanding and preventing necrotizing enterocolitis. Curr. Gastroenterol. Rep. 10, 450–457 (2008).
Nguyen, D. N. et al. Prenatal intra-amniotic endotoxin induces fetal gut and lung immune responses and postnatal systemic inflammation in preterm pigs. Am. J. Pathol. 188, 2629–2643 (2018).
Wolfs, T. G. et al. IL-1alpha mediated chorioamnionitis induces depletion of FoxP3+ cells and ileal inflammation in the ovine fetal gut. PLoS One 6, e18355 (2011).
Wolfs, T. G. et al. Endotoxin induced chorioamnionitis prevents intestinal development during gestation in fetal sheep. PLoS One 4, e5837 (2009).
Fricke, E. M. et al. Lipopolysaccharide-induced maternal inflammation induces direct placental injury without alteration in placental blood flow and induces a secondary fetal intestinal injury that persists into adulthood. Am. J. Reprod. Immunol. 79, e12816 (2018).
Yan, X., Managlia, E., Tan, X. D. & De Plaen, I. G. Prenatal inflammation impairs intestinal microvascular development through a TNF-dependent mechanism and predisposes newborn mice to necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G57–G66 (2019).
Leviton, A. et al. The clustering of disorders in infants born before the 28th week of gestation. Acta Paediatr. 99, 1795–1800 (2010).
Nelson, D. B., McIntire, D. D. & Leveno, K. J. Reply. Am. J. Obstet. Gynecol. 218, 360–362 (2018).
Leveno, K. J., McIntire, D. D., Bloom, S. L., Sibley, M. R. & Anderson, R. J. Decreased preterm births in an inner-city public hospital. Obstet. Gynecol. 113, 578–584 (2009).
Kaiser, J. R., Tilford, J. M., Simpson, P. M., Salhab, W. A. & Rosenfeld, C. R. Hospital survival of very-low-birth-weight neonates from 1977 to 2000. J. Perinatol. 24, 343–350 (2004).
Coursey, C. A. et al. Radiologists’ agreement when using a 10-point scale to report abdominal radiographic findings of necrotizing enterocolitis in neonates and infants. Am. J. Roentgenol. 191, 190–197 (2008).
Martin, C. R., Bellomy, M., Allred, E. N., Fichorova, R. N. & Leviton, A. Systemic inflammation associated with severe intestinal injury in extremely low gestational age newborns. Fetal Pediatr. Pathol. 32, 222–234 (2013).
Acknowledgements
We wish to thank several people who participated in data collection: Patti J Burchfield, RN, Pollieanna M Sepulveda, RN and Anita Thomas, RN.
Funding
This work was supported by Children’s Clinical Research Advisory Council (CCRAC) grant awarded to I.N.M.
Author information
Authors and Affiliations
Contributions
I.N.M. wrote the first version of the manuscript. L.P.B. and S.B. performed statistical analyses. All authors participated in the study design, data collection, data interpretation, and revision and approval of the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mir, I.N., Sánchez-Rosado, M., Reis, J. et al. Impact of fetal inflammatory response on the severity of necrotizing enterocolitis in preterm infants. Pediatr Res 95, 1308–1315 (2024). https://doi.org/10.1038/s41390-023-02942-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02942-2
This article is cited by
-
Fetal inflammatory response spectrum: mapping its impact on severity of necrotising enterocolitis
Pediatric Research (2023)