Abstract
Background
Irritable bowel syndrome is common in children and exhibits a high placebo response. This study was to explore the placebo response rate and its influencing factors in children with irritable bowel syndrome.
Methods
A systematic search was performed on Pubmed, Embase, MEDLINE, Cochrane Library, CNKI, Wanfang, and CBM from database inception to March 2022. Randomized controlled trials of irritable bowel syndrome in children were included in the study. The primary outcome was the placebo response rate of improvement.
Results
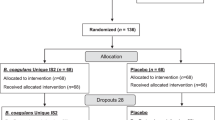
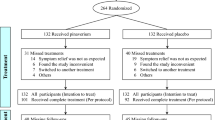
Thirteen studies were included, with 445 patients in the placebo group. The rate of improvement and abdominal pain disappearance were 28.2% (95% CI, 16.6–39.9%) and 5% (95% CI, 0–18.4%). The placebo response based on the abdominal pain score was 0.675 (95% CI, 0.203–1.147). The mode of administration (P < 0.01), dosing schedule (P < 0.01), and clinical outcome assessor (P = 0.04) have a significant impact on the magnitude of placebo effect.
Conclusions
The placebo response rate for pediatric irritable bowel syndrome was 28.2%. In clinical trials, reducing dosing frequency, selecting appropriate dosage forms, and using patient-reported outcomes can help mitigate the placebo effect.
Impact
-
This is the first meta-analysis to assess the placebo response rates for improvement and disappearance in children with IBS.
-
The finding suggested that the mode of administration, dosing schedule, and clinical outcome assessor could potentially influence the magnitude of the placebo effect in children with IBS.
-
This study would provide a basis for estimating sample size in clinical trial design with a placebo control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The raw data and code can be obtained directly from the author.
References
Drossman, D. A. ROME IV: Functional Gastrointestinal Disorders/Disorders of Gut-Brain Interaction (The Rome Foundation, Raleigh, 2016).
Korterink, J. J., Diederen, K., Benninga, M. A. & Tabbers, M. M. Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PLoS ONE 10, e0126982 (2015).
Devanarayana, N. M. et al. Epidemiology of irritable bowel syndrome in children and adolescents in Asia. J. Pediatr. Gastroenterol. Nutr. 60, 792–798 (2015).
Thapar, N. et al. Paediatric functional abdominal pain disorders. Nat. Rev. Dis. Prim. 6, 89 (2020).
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for industry irritable bowel syndrome-clinical evaluation of drugs for treatment. https://www.fda.gov/media/78622/download (2012).
European Medicines Agency. Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome. https://www.ema.europa.eu/en/evaluation-medicinal-products-treatment-irritable-bowel-syndrome (2014).
China Food and Drug Administration. Clinical Research Guidance for New Drug of Chinese Medicine in Irritable Bowel Syndrome 2017. https://www.cde.org.cn/zdyz/domesticinfopage?zdyzIdCODE=c2b78e36b58535dfb0a1150be64d9470 (2017).
Lacy, B. E. et al. ACG clinical guideline: management of irritable bowel syndrome. Am. J. Gastroenterol. 116, 17–44 (2021).
Saps, M. et al. Recommendations for pharmacological clinical trials in children with irritable bowel syndrome: the Rome foundation pediatric subcommittee on clinical trials. Neurogastroenterol. Motil. 28, 1619–1631 (2016).
Munnangi, S., Sundjaja, J. H., Singh, K., Dua, A. & Angus, L. D. Placebo effect. In StatPearls (StatPearls Publishing, Treasure Island, FL, 2022).
Colloca, L. The placebo effect in pain therapies. Annu Rev. Pharmacol. Toxicol. 59, 191–211 (2019). Jan 6.
Pitz, M., Cheang, M. & Bernstein, C. N. Defining the predictors of the placebo response in irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 3, 237–247 (2005).
Patel, S. M. et al. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol. Motil. 17, 332–340 (2005).
Dorn, S. D. et al. A meta-analysis of the placebo response in complementary and alternative medicine trials of irritable bowel syndrome. Neurogastroenterol. Motil. 19, 630–637 (2007).
Ford, A. C. & Moayyedi, P. Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther. 32, 144–158 (2010).
Barberio, B., Savarino, E. V., Black, C. J. & Ford, A. C. Placebo response rates in trials of licensed drugs for irritable bowel syndrome with constipation or diarrhea: meta-analysis. Clin. Gastroenterol. Hepatol. S1542-3565, 00905–00908 (2021).
Bosman, M. et al. The placebo response rate in pharmacological trials in patients with irritable bowel syndrome: a systematic review and meta-analysis[J]. Lancet Gastroenterol. Hepatol. 6, 459–473 (2021).
Hoekman, D. R. et al. The placebo response in pediatric abdominal pain-related functional gastrointestinal disorders: a systematic review and meta-analysis. J. Pediatr. 182, 155–163.e7 (2017).
Hyams, J. S. et al. Functional disorders: children and adolescents. Gastroenterology S0016-5085, 00181–00185 (2016).
Rajindrajith, S., Zeevenhooven, J., Devanarayana, N. M., Perera, B. J. C. & Benninga, M. A. Functional abdominal pain disorders in children. Expert Rev. Gastroenterol. Hepatol. 12, 369–390 (2018).
Enck, P. & Klosterhalfen, S. Placebos and the placebo effect in drug trials. Handb. Exp. Pharmacol. 260, 399–431 (2019).
Sterne, J. A. C. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898 (2019).
Luo, M. et al. Meta analysis of single rate in R software. J. Evid. Based Med. 13, 181–184 (2013).
Kline, R. M., Kline, J. J., Di Palma, J. & Barbero, G. J. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J. Pediatr. 138, 125–128 (2001).
Bauserman, M. & Michail, S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J. Pediatr. 147, 197–201 (2005).
Gawrońska, A., Dziechciarz, P., Horvath, A. & Szajewska, H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol. Ther. 25, 177–184 (2007).
Bahar, R. J., Collins, B. S., Steinmetz, B. & Ament, M. E. Double-blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J. Pediatr. 152, 685–689 (2008).
Handen, B. L. et al. A double-blind, placebo-controlled trial of oral human immunoglobulin for gastrointestinal dysfunction in children with autistic disorder. J. Autism Dev. Disord. 39, 796–805 (2009).
Francavilla, R. et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics 126, e1445–e1452 (2010).
Guandalini, S. et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J. Pediatr. Gastroenterol. Nutr. 51, 24–30 (2010).
Romano, C., Comito, D., Famiani, A., Calamarà, S. & Loddo, I. Partially hydrolyzed guar gum in pediatric functional abdominal pain. World J. Gastroenterol. 19, 235–240 (2013).
Kianifar, H. et al. Probiotic for irritable bowel syndrome in pediatric patients: a randomized controlled clinical trial. Electron Physician 7, 1255–1260 (2015).
Shulman, R. J. et al. Psyllium fiber reduces abdominal pain in children with irritable bowel syndrome in a randomized, double-blind trial. Clin. Gastroenterol. Hepatol. 15, 712–719.e4 (2017).
Shulman, R. J. et al. Randomized, double blind trial of psyllium fiber in children with irritable bowel syndrome (IBS). Gastroenterology 148, S120 (2015).
Giannetti, E. et al. A mixture of 3 bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome: a multicenter, randomized, double-blind, placebo-controlled, crossover trial. J. Clin. Gastroenterol. 51, e5–e10 (2017).
Sudha, M. R., Jayanthi, N., Aasin, M., Dhanashri, R. D. & Anirudh, T. Efficacy of Bacillus coagulans unique IS2 in treatment of irritable bowel syndrome in children: a double blind, randomised placebo controlled study. Benef. Microbes 9, 563–572 (2018).
Rahmani, P., Ghouran-Orimi, A., Motamed, F. & Moradzadeh, A. Evaluating the effects of probiotics in pediatrics with recurrent abdominal pain. Clin. Exp. Pediatr. 63, 485–490 (2020).
Schober, P., Mascha, E. J. & Vetter, T. R. Statistics from A (Agreement) to Z (z Score): a guide to interpreting common measures of association, agreement, diagnostic accuracy, effect size, heterogeneity, and reliability in medical research. Anesth. Analg. 133, 1633–1641 (2021).
Weimer, K. et al. Placebo effects in children: a review. Pediatr. Res. 74, 96–102 (2013).
Janiaud, P. et al. Is the perceived placebo effect comparable between adults and children? A meta-regression analysis. Pediatr. Res. 81, 11–17 (2017).
Gniß, S., Kappesser, J. & Hermann, C. Placebo effect in children: the role of expectation and learning. Pain 161, 1191–1201 (2020).
Mohammad, S., Pusatcioglu, C. & Saps, M. Comparison of primary efficacy endpoints recommended by regulatory agencies in children with functional gastrointestinal disorders. Gastroenterology 148, S–586 (2015)..
Nurko, S. et al. Effect of open-label placebo on children and adolescents with functional abdominal pain or irritable bowel syndrome: a randomized clinical trial. JAMA Pediatr. 176, 349–356 (2022).
Author information
Authors and Affiliations
Contributions
L.L.C. and X.L. contributed equally to this manuscript and should be considered joint first author. L.L.C. and X.L. carried out the concept, design and drafting of the manuscript, performed the acquisition, analysis, and interpretation of data, and critically revised the manuscript for important intellectual content. Q.H.C. and S.Y.H. carried out the concept, design and drafting of the manuscript. G.S.X. and Y.Z. performed the Statistical analysis. WCS and Z.H.Z. searched databases, screened articles, and extracted data. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, LL., Li, X., Cai, QH. et al. Irritable bowel syndrome in children: the placebo response rate and influencing factors a meta-analysis. Pediatr Res (2024). https://doi.org/10.1038/s41390-023-02996-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-023-02996-2