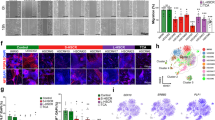
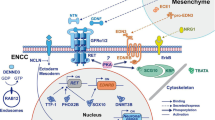
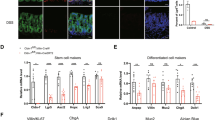
Abstract
Background
Hirschsprung disease (HSCR) is a congenital intestinal disease characterised by functional obstruction of the colon. Herein, we investigated the role and mechanism of the gene GFRA4 in HSCR.
Methods
GFRA4 expression in the ganglionic and aganglionic segment tissues in patients with HSCR and healthy colon tissues were detected using qRT-PCR, western blot, and immunohistochemistry. Cell proliferation, cycle distribution, apoptosis, changes in mitochondrial membrane potential, and differentiation were assessed in mouse enteric neural crest stem cells (ENCSCs) using the CCK-8 assay, EdU staining, flow cytometry, JC-1 probe, and immunofluorescence, respectively. GSEA analysis was performed to screen the signaling pathways regulated by GFRA4.
Results
GFRA4 was downregulated in aganglionic segment tissues compared to control and ganglionic segment tissues. GFRA4 overexpression promoted proliferation and differentiation, and inhibited apoptosis in ENCSCs, while GFRA4 down-regulation had the opposite result. GFRA4 activated the hedgehog pathway. GFRA4 overexpression enhanced the expression of key factors of the hedgehog pathway, including SMO, SHH, and GLI1. However, GFRA4 down-regulation reduced their expression. An antagonist of hedgehog pathway, cyclopamine, attenuated the effect of GFRA4 overexpression on proliferation, differentiation, and apoptosis of ENCSCs.
Conclusion
GFRA4 promotes proliferation and differentiation but inhibits apoptosis of ENCSCs via the hedgehog pathway in HSCR.
Impact
-
This study confirms that GFRA4 improves the proliferation and differentiation of ENCSCs via modulation of the hedgehog pathway.
-
This study for the first time revealed the role and the mechanism of the action of GFRA4 in HSCR, which indicates that GFRA4 may play a role in the pathological development of HSCR.
-
Our findings may lay the foundation for further investigation of the mechanisms underlying HSCR development and into targets of HSCR treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Butler Tjaden, N. E. & Trainor, P. A. The developmental etiology and pathogenesis of Hirschsprung disease. Transl. Res. 162, 1–15 (2013).
Muise, E. D. & Cowles, R. A. Rectal biopsy for Hirschsprung’s disease: a review of techniques, pathology, and complications. World J. Pediatr.: WJP 12, 135–141 (2016).
Gunadi et al. Long-term functional outcomes of patients with Hirschsprung disease following pull-through. BMC Pediatr. 22, 022–03301 (2022).
Xia, R. P. et al. Circ-ITCH overexpression promoted cell proliferation and migration in Hirschsprung disease through miR-146b-5p/RET axis. Pediatr. Res. 92, 1008–1016 (2022).
Kyrklund, K. et al. ERNICA guidelines for the management of rectosigmoid Hirschsprung’s disease. Orphanet J. Rare Dis. 15, 020–01362 (2020).
Soret, R. et al. Genetic Background Influences Severity of Colonic Aganglionosis and Response to GDNF Enemas in the Holstein Mouse Model of Hirschsprung Disease. Int. J. Mol. Sci. 22, 13140 (2021).
Jain, S. et al. Organotypic specificity of key RET adaptor-docking sites in the pathogenesis of neurocristopathies and renal malformations in mice. J. Clin. Invest. 120, 778–790 (2010).
Souza, R. P. et al. Genetic association of the GDNF alpha-receptor genes with schizophrenia and clozapine response. J. Psychiatr. Res. 44, 700–706 (2010).
Enokido, Y. et al. GFR alpha-4 and the tyrosine kinase Ret form a functional receptor complex for persephin. Curr. Biol. 8, 1019–1022 (1998).
Yang, J., Runeberg-Roos, P., Leppänen, V. M. & Saarma, M. The mouse soluble GFRalpha4 receptor activates RET independently of its ligand persephin. Oncogene 26, 3892–3898 (2007).
Lee, K. et al. Proteome-wide discovery of mislocated proteins in cancer. Genome Res. 23, 1283–1294 (2013).
Ngan, E. S. et al. Hedgehog/Notch-induced premature gliogenesis represents a new disease mechanism for Hirschsprung disease in mice and humans. J. Clin. Investig. 121, 3467–3478 (2011).
Du, C. et al. Apoptotic neuron-secreted HN12 inhibits cell apoptosis in Hirschsprung’s disease. Int J. Nanomed. 11, 5871–5881 (2016).
Xie, H. et al. Long none coding RNA HOTTIP/HOXA13 act as synergistic role by decreasing cell migration and proliferation in Hirschsprung disease. Biochem Biophys. Res Commun. 463, 569–574 (2015).
Wu, F. et al. MPGES-1 derived PGE2 inhibits cell migration by regulating ARP2/3 in the pathogenesis of Hirschsprung disease. J. Pediatr. Surg. 54, 2032–2037 (2019).
Chen, G. et al. MicroRNA-939 inhibits cell proliferation via targeting LRSAM1 in Hirschsprung’s disease. Aging 9, 2471–2479 (2017).
Lake, J. I. & Heuckeroth, R. O. Enteric nervous system development: migration, differentiation, and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 305, 2 (2013).
Langer, J. C. Hirschsprung disease. Curr. Opin. Pediatr. 25, 368–374 (2013).
Shu, X. et al. Treatment of aganglionic megacolon mice via neural stem cell transplantation. Mol. Neurobiol. 48, 429–437 (2013).
McKeown, S. J., Stamp, L., Hao, M. M. & Young, H. M. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley Interdiscip. Rev. Dev. Biol. 2, 113–129 (2013).
Wallace, A. S. et al. Inhibition of cell death results in hyperganglionosis: implications for enteric nervous system development. Neurogastroenterol. Motil. 21, 768–e49 (2009).
Wang, G. et al. Downregulation of microRNA-483-5p Promotes Cell Proliferation and Invasion by Targeting GFRA4 in Hirschsprung’s Disease. DNA Cell Biol. 36, 930–937 (2017).
Wang, G. et al. Demethylation of GFRA4 Promotes Cell Proliferation and Invasion in Hirschsprung Disease. DNA Cell Biol. 37, 316–324 (2018).
Yang, S. et al. Sesamin induces A549 cell mitophagy and mitochondrial apoptosis via a reactive oxygen species-mediated reduction in mitochondrial membrane potential. Korean J. Physiol. Pharm. 24, 223–232 (2020).
Zhang, B., Bian, W., Pal, A. & He, Y. Macrophage apoptosis induced by aqueous C60 aggregates changing the mitochondrial membrane potential. Environ. Toxicol. Pharm. 39, 237–246 (2015).
Thomas, A. L. et al. Autologous Transplantation of Skin-Derived Precursor Cells in a Porcine Model. J. Pediatr. Surg. 55, 194–200 (2020).
Rollo, B. N. et al. Enteric Neural Cells From Hirschsprung Disease Patients Form Ganglia in Autologous Aneuronal Colon. Cell Mol. Gastroenterol. Hepatol. 2, 92–109 (2015).
Cooper, J. E. et al. In Vivo Transplantation of Enteric Neural Crest Cells into Mouse Gut; Engraftment, Functional Integration and Long-Term Safety. PLoS One 11, e0147989 (2016).
Cooper, J. E. et al. In vivo transplantation of fetal human gut-derived enteric neural crest cells. Neurogastroenterol. Motil. 29, 6 (2017).
Kato, H. et al. Immunocytochemical characterization of supporting cells in the enteric nervous system in Hirschsprung’s disease. J. Pediatr. Surg. 25, 514–519 (1990).
Ingham, P. W. Hedgehog signaling. Curr. Top. Develop. Biol. 149, 1–58 (2022).
Lau, S. T. et al. Activation of Hedgehog Signaling Promotes Development of Mouse and Human Enteric Neural Crest Cells, Based on Single-Cell Transcriptome Analyses. Gastroenterology 157, 1556–1571 (2019).
Ellis, T. et al. Patched 1 conditional null allele in mice. Genesis 36, 158–161 (2003).
Fattahi, F. et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature 531, 105–109 (2016).
Funding
Project ZR2020MH048 supported by Shandong Provincial Natural Science Foundation and the Natural Science Foundation of Tibet Autonomous Region (No.XZ202101ZR0004G)
Author information
Authors and Affiliations
Contributions
Conception and design: F.F.Z.; Perform research: F.F.Z., L.J.Z., and M.Y.C.; Data analysis and interpretation: B.Z.M., F.G., and G.W.; Manuscript writing: All authors; Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, F., Cui, M., Zhang, L. et al. GFRA4 improves the neurogenic potential of enteric neural crest stem cells via hedgehog pathway. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03158-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03158-8