Abstract
Background
Early and precise diagnosis of bronchopulmonary dysplasia (BPD) is essential to improve the prognosis of preterm infants with BPD. Studying ferroptosis-related genes for diagnostic markers of BPD was the objective of this study.
Methods
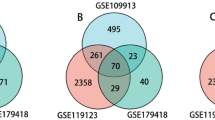
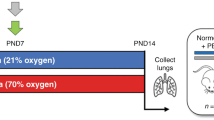
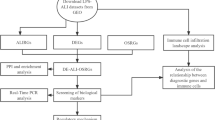
Using the GEO database and the FerrDb database, we obtained the GSE32472 dataset and screened the ferroptosis-related differentially expressed mRNAs (FRDE-mRNAs). By using Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG), possible biological functions and pathways were identified for FRDE-mRNAs. Three machine learning algorithms (LASSO, SVM-RFE, Random Forest) were used to recognize hub genes, as well as CIBERSORT for exploring the immune landscape of BPD and controls. Functional predictions for hub genes were made using single-gene gene set enrichment analysis (GSEA).
Results
Twenty three FRDE-mRNAs were obtained and were mainly involved in autophagy, fatty acid metabolism and ferroptosis. The four hub genes (LPIN1, ACADSB, WIPI1 and SLC7A11) screened were utilized to construct a diagnostic nomogram. The receiver operating characteristic (ROC) curves and calibration curves demonstrateld that the nomogram exhibited good predictive performance. Eight types of immune cell markers differed significantly between BPD and controls.
Conclusion
We developed a diagnostic model for BPD, which could facilitate the early diagnosis and timely intervention of BPD.
Impact
-
The role of ferroptosis in bronchopulmonary dysplasia is rarely reported.
-
The ferroptosis-related genes (LPIN1, ACADSB, WIPI1 and SLC7A11) we identified could serve as early diagnostic biomarkers for BPD.
-
Immune cell infiltration features in BPD and signaling pathways associated with marker genes give new insight into the disease process and provide a basis for further research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Publicly available dataset was analyzed in this study. The dataset can be found here: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE32472.
References
Lui, K. et al. Inter-center variability in neonatal outcomes of preterm infants: a longitudinal evaluation of 298 neonatal units in 11 countries. Semin Fetal Neonatal Med. 26, 101196 (2021).
Cheong, J. L. Y. & Doyle, L. W. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin Perinatol. 42, 478–484 (2018).
Prayle, A. & Rosenow, T. Looking under the bonnet of bronchopulmonary dysplasia with MRI. Thorax. 75, 100 (2020).
Chen, X., Kang, R., Kroemer, G. & Tang, D. Organelle-specific regulation of ferroptosis. Cell Death Differ. 28, 2843–2856 (2021).
Mou, Y. et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 12, 34 (2019).
Yu, S. et al. Recent progress of ferroptosis in lung diseases. Front Cell Dev Biol. 9, 789517 (2021).
Yoshida, M. et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat Commun. 10, 3145 (2019).
Li, X. et al. Ferroptosis inhibitor alleviates radiation-induced lung fibrosis (RILF) via down-regulation of TGF-Β1. J Inflamm. 16, 11 (2019).
Chou, H. C. & Chen, C. M. Hyperoxia induces ferroptosis and impairs lung development in neonatal mice. Antioxidants. 11, 641 (2022).
Pietrzyk, J. J. et al. Gene expression profiling in preterm infants: new aspects of bronchopulmonary dysplasia development. PloS One. 8, e78585 (2013).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 12, 453–457 (2015).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 163, 1723–1729 (2001).
Wang, X. et al. Lipocalin-2 silencing suppresses inflammation and oxidative stress of acute respiratory distress syndrome by ferroptosis via inhibition of MAPK/ERK pathway in neonatal mice. Bioengineered. 13, 508–520 (2022).
Yu, T., Yu, Y., Ma, Y. & Chen, G. Inhibition of CREB promotes glucocorticoids action on airway inflammation in pediatric asthma by promoting ferroptosis of eosinophils. Allergol Immunopathol. 51, 164–174 (2023).
Dylag, A. M. et al.New insights into the natural history of bronchopulmonary dysplasia from proteomics and multiplexed immunohistochemistry. Am J Physiol Lung Cell Mol Physiol. 325, L419–L433 (2023).
Moreira, A. et al. Development of a peripheral blood transcriptomic gene signature to predict bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 324, L76–L87 (2023).
Yeganeh, B. et al. Autophagy is required for lung development and morphogenesis. J Clin Investig. 129, 2904–2919 (2019).
Li, X. et al. Autophagy reprograms alveolar progenitor Cell metabolism in response to lung injury. Stem Cell Rep. 14, 420–432 (2020).
Yao, H. et al. Fatty acid oxidation protects against hyperoxia-induced endothelial cell apoptosis and lung injury in neonatal mice. Am J Respir Cell Mol Biol. 60, 667–677 (2019).
Peterson, A. L., Carr, J. F., Ji, X., Dennery, P. A. & Yao, H. Hyperoxic exposure caused lung lipid compositional changes in neonatal mice. Metabolites. 10, 340 (2020).
Das, U. N. Saturated fatty acids, MUFAs and PUFAs regulate ferroptosis. Cell Chem Biol. 26, 309–311 (2019).
Magtanong, L. et al. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem Biol. 26, 420–432.e429 (2019).
Liu, J. et al. Autophagy-dependent ferroptosis: machinery and regulation.Cell Chem Biol. 27, 420–435 (2020).
Tang, S. Q., Jiang, Q. Y., Yang, C. F., Zou, X. T. & Dong, X. Y. [Research and development of Lipin family]. Yi Chuan 32, 981–993 (2010).
Chen, Y., Rui, B. B., Tang, L. Y. & Hu, C. M. Lipin family proteins-key regulators in lipid metabolism. Ann Nutr Metab. 66, 10–18 (2015).
Yue, L., Lu, X., Dennery, P. A. & Yao, H. Metabolic dysregulation in bronchopulmonary dysplasia: implications for identification of biomarkers and therapeutic approaches. Redox Biol. 48, 102104 (2021).
Jiang, P. et al. Transcriptomic analysis of short/branched-chain acyl-coenzyme A dehydrogenase knocked out BMECs Revealed Its Regulatory Effect On Lipid Metabolism. Front Vet Sci. 8, 744287 (2021).
Lu, D. et al. ACADSB regulates ferroptosis and affects the migration, invasion, and proliferation of colorectal cancer cells. Cell Biol Int. 44, 2334–2343 (2020).
Koppula, P., Zhang, Y., Zhuang, L. & Gan, B. Amino acid transporter SLC7A11/XCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 38, 12 (2018).
Wang, S. J. et al. Acetylation is crucial for P53-mediated ferroptosis and tumor suppression. Cell Rep. 17, 366–373 (2016).
Dong, H. et al. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging. 12, 12943–12959 (2020).
Almannai, M., Marafi, D. & El-Hattab, A. W.WIPI proteins: biological functions and related syndromes. Front Mol Neurosci. 15, 1011918 (2022).
Kumar, V. H. S., Wang, H. & Nielsen, L. Adaptive immune responses are altered in adult mice following neonatal hyperoxia. Physiol Rep. 6, e13577 (2018).
Yildiz, C.Mechanical ventilation induces neutrophil extracellular trap formation. Anesthesiol. 122, 864–875 (2015).
Zhang, Z. et al. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HUR to regulate ferroptosis in hepatic stellate cells. Autophagy. 14, 2083–2103 (2018).
Kalymbetova, T. V. et al. Resident alveolar macrophages are master regulators of arrested alveolarization in experimental bronchopulmonary dysplasia. J Pathol. 245, 153–159 (2018).
Author information
Authors and Affiliations
Contributions
Changjiang Fang designed the research, analyzed the data, and wrote the manuscript; Haixia Tu, Rong Li and Dengqin Bi analyzed and interpreted the data; Guihua Shu designed the research, analyzed the data, and corrected the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, C., Tu, H., Li, R. et al. Bronchopulmonary dysplasia: analysis and validation of ferroptosis-related diagnostic biomarkers and immune cell infiltration features. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03249-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03249-6