Abstract
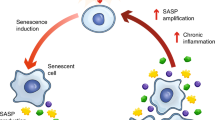
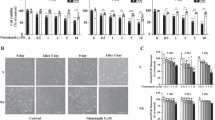
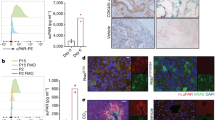
Cellular senescence is a stress-induced, stable cell cycle arrest phenotype which generates a pro-inflammatory microenvironment, leading to chronic inflammation and age-associated diseases. Determining the fundamental molecular pathways driving senescence instead of apoptosis could enable the identification of senolytic agents to restore tissue homeostasis. Here, we identify thrombomodulin (THBD) signaling as a key molecular determinant of the senescent cell fate. Although normally restricted to endothelial cells, THBD is rapidly upregulated and maintained throughout all phases of the senescence program in aged mammalian tissues and in senescent cell models. Mechanistically, THBD activates a proteolytic feed-forward signaling pathway by stabilizing a multi-protein complex in early endosomes, thus forming a molecular basis for the irreversibility of the senescence program and ensuring senescent cell viability. Therapeutically, THBD signaling depletion or inhibition using vorapaxar, an FDA-approved drug, effectively ablates senescent cells and restores tissue homeostasis in liver fibrosis models. Collectively, these results uncover proteolytic THBD signaling as a conserved pro-survival pathway essential for senescent cell viability, thus providing a pharmacologically exploitable senolytic target for senescence-associated diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA transcriptional profiling data involved in this study has been uploaded to NCBI GEO datasets (GSE228941).
References
Collado, M., Blasco, M. A. & Serrano, M. Cellular senescence in cancer and aging. Cell 130, 223–233 (2007).
Campisi, J. Cancer, aging and cellular senescence. In Vivo 14, 183–188 (2000).
Rodier, F. & Campisi, J. Four faces of cellular senescence. J. Cell Biol. 192, 547–556 (2011).
Campisi, J. & d’Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007).
Childs, B. G. et al. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21, 1424–1435 (2015).
He, S. & Sharpless, N. E. Senescence in health and disease. Cell 169, 1000–1011 (2017).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Johmura, Y. et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science 371, 265–270 (2021).
Justice, J. N. et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563 (2019).
Zhang, L. et al. Recent advances in the discovery of senolytics. Mech. Ageing Dev. 200, 111587 (2021).
Carpenter, V. J., Saleh, T. & Gewirtz, D. A. Senolytics for cancer therapy: is all that glitters really gold? Cancers (Basel) 13, 723 (2021).
Kovacovicova, K. et al. Senolytic cocktail Dasatinib+Quercetin (D+Q) does not enhance the efficacy of senescence-inducing chemotherapy in liver cancer. Front. Oncol. 8, 459 (2018).
Suvarna, V., Singh, V. & Murahari, M. Current overview on the clinical update of Bcl-2 anti-apoptotic inhibitors for cancer therapy. Eur. J. Pharmacol. 862, 172655 (2019).
Gasek, N. S. et al. Strategies for targeting senescent cells in human disease. Nat. Aging 1, 870–879 (2021).
Hernandez-Segura, A. et al. Unmasking transcriptional heterogeneity in senescent cells. Curr. Biol. 27, 2652–2660.e4 (2017).
Aird, K. M. & Zhang, R. Detection of senescence-associated heterochromatin foci (SAHF). Methods Mol. Biol. 965, 185–196 (2013).
Kosar, M. et al. Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type- and insult-dependent manner and follow expression of p16(ink4a). Cell Cycle 10, 457–468 (2011).
Martin, F. A., Murphy, R. P. & Cummins, P. M. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am. J. Physiol. Heart Circ. Physiol. 304, H1585–H1597 (2013).
Okamoto, T. et al. Thrombomodulin: a bifunctional modulator of inflammation and coagulation in sepsis. Crit. Care Res. Pract. 2012, 614545 (2012).
Chao, T. H. et al. Soluble thrombomodulin is a paracrine anti-apoptotic factor for vascular endothelial protection. Int. J. Cardiol. 172, 340–349 (2014).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Alexander, P. B. et al. EGF promotes mammalian cell growth by suppressing cellular senescence. Cell Res. 25, 135–138 (2015).
Yuan, L. et al. Switching off IMMP2L signaling drives senescence via simultaneous metabolic alteration and blockage of cell death. Cell Res. 28, 625–643 (2018).
Xiang, H. et al. UHRF1 is required for basal stem cell proliferation in response to airway injury. Cell Discov. 3, 17019 (2017).
Chong, M. et al. CD36 initiates the secretory phenotype during the establishment of cellular senescence. EMBO Rep. 19, e45274 (2018).
Cheng, Y. et al. Intraovarian thrombin and activated protein C signaling system regulates steroidogenesis during the periovulatory period. Mol. Endocrinol. 26, 331–340 (2012).
Tsiang, M., Lentz, S. R. & Sadler, J. E. Functional domains of membrane-bound human thrombomodulin. EGF-like domains four to six and the serine/threonine-rich domain are required for cofactor activity. J. Biol. Chem. 267, 6164–6170 (1992).
Wolter, J. et al. Thrombomodulin-dependent protein C activation is required for mitochondrial function and myelination in the central nervous system. J. Thromb. Haemost. 14, 2212–2226 (2016).
van Deursen, J. M. The role of senescent cells in ageing. Nature 509, 439–446 (2014).
Yousefzadeh, M. J. et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 19, e13094 (2020).
Wootten, D. et al. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 19, 638–653 (2018).
Mosnier, L. O. et al. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood 120, 5237–5246 (2012).
Isermann, B. et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat. Med. 13, 1349–1358 (2007).
Riewald, M. et al. Activated protein C signals through the thrombin receptor PAR1 in endothelial cells. J. Endotoxin. Res. 9, 317–321 (2003).
Cheng, T. et al. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 9, 338–342 (2003).
McMahon, H. T. & Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533 (2011).
Hanyaloglu, A. C. & von Zastrow, M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 (2008).
Sungkaworn, T. et al. Single-molecule imaging reveals receptor-G protein interactions at cell surface hot spots. Nature 550, 543–547 (2017).
Finn, R. S. et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375, 1925–1936 (2016).
Finn, R. S. et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res. 18, 67 (2016).
Goel, S. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475 (2017).
Grimsey, N. J. et al. Recycling and endosomal sorting of protease-activated receptor-1 is distinctly regulated by Rab11A and Rab11B proteins. J. Biol. Chem. 291, 2223–2236 (2016).
Trejo, J., Hammes, S. R. & Coughlin, S. R. Termination of signaling by protease-activated receptor-1 is linked to lysosomal sorting. Proc. Natl. Acad. Sci. USA 95, 13698–13702 (1998).
Lopez-Otin, C. et al. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Takenaka, Y. et al. Prolonged disturbance of proteostasis induces cellular senescence via temporal mitochondrial dysfunction and subsequent mitochondrial accumulation in human fibroblasts. FEBS J. 289, 1650–1667 (2022).
Joy, J. et al. Proteostasis failure and mitochondrial dysfunction leads to aneuploidy-induced senescence. Dev. Cell 56, 2043–2058.e7 (2021).
Saez, I. & Vilchez, D. The mechanistic links between proteasome activity, aging and age-related diseases. Curr. Genomics 15, 38–51 (2014).
Kaushik, S. & Cuervo, A. M. Proteostasis and aging. Nat. Med. 21, 1406–1415 (2015).
Coffey, E. E. et al. Lysosomal alkalization and dysfunction in human fibroblasts with the Alzheimer’s disease-linked presenilin 1 A246E mutation can be reversed with cAMP. Neuroscience 263, 111–124 (2014).
Wang, L. et al. A pH probe inhibits senescence in mesenchymal stem cells. Stem Cell Res. Ther. 9, 343 (2018).
Sun, Y. et al. Lysosome activity is modulated by multiple longevity pathways and is important for lifespan extension in C. elegans. Elife 9, e55745 (2020).
Wandrer, F. et al. Senescence mirrors the extent of liver fibrosis in chronic hepatitis C virus infection. Aliment Pharmacol. Ther. 48, 270–280 (2018).
Wiemann, S. U. et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 16, 935–942 (2002).
Aravinthan, A. et al. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J. Hepatol. 58, 549–556 (2013).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Ogrodnik, M. et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 8, 15691 (2017).
Ritschka, B. et al. The senotherapeutic drug ABT-737 disrupts aberrant p21 expression to restore liver regeneration in adult mice. Genes Dev. 34, 489–494 (2020).
Dolgin, E. Publisher correction: send in the senolytics. Nat. Biotechnol. 39, 1308 (2021).
Beerman, I., Basisty, N. & de Cabo, R. Short-term senolytic treatment: a paradigm to promote fracture repair during aging. J. Clin. Invest. 132, e158871 (2022).
Fleury, H. et al. Exploiting interconnected synthetic lethal interactions between PARP inhibition and cancer cell reversible senescence. Nat. Commun. 10, 2556 (2019).
Sharma, A. K. et al. The senolytic drug Navitoclax (ABT-263) causes trabecular bone loss and impaired osteoprogenitor function in aged mice. Front. Cell Dev. Biol. 8, 354 (2020).
Munro, J. et al. Histone deacetylase inhibitors induce a senescence-like state in human cells by a p16-dependent mechanism that is independent of a mitotic clock. Exp. Cell Res. 295, 525–538 (2004).
Lyu, G. et al. TGF-beta signaling alters H4K20me3 status via miR-29 and contributes to cellular senescence and cardiac aging. Nat. Commun. 9, 2560 (2018).
Liu, P. et al. m(6)A-independent genome-wide METTL3 and METTL14 redistribution drives the senescence-associated secretory phenotype. Nat. Cell Biol. 23, 355–365 (2021).
Tian, Y. et al. Loss of PTEN induces lung fibrosis via alveolar epithelial cell senescence depending on NF-kappaB activation. Aging Cell 18, e12858 (2019).
Palfy, M., Remenyi, A. & Korcsmaros, T. Endosomal crosstalk: meeting points for signaling pathways. Trends Cell Biol. 22, 447–456 (2012).
Murphy, J. E. et al. Endosomes: a legitimate platform for the signaling train. Proc. Natl Acad. Sci. USA 106, 17615–17622 (2009).
Cullen, P. J. & Steinberg, F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 19, 679–696 (2018).
Grimsey, N., Lin, H. & Trejo, J. Endosomal signaling by protease-activated receptors. Methods Enzymol. 535, 389–401 (2014).
Chen, B. et al. Adaptor protein complex-2 (AP-2) and epsin-1 mediate protease-activated receptor-1 internalization via phosphorylation- and ubiquitination-dependent sorting signals. J. Biol. Chem. 286, 40760–40770 (2011).
Chen, B. et al. Regulation of protease-activated receptor 1 signaling by the adaptor protein complex 2 and R4 subfamily of regulator of G protein signaling proteins. J. Biol. Chem. 289, 1580–1591 (2014).
Baker, N. et al. Vacuolar ATPase depletion affects mitochondrial ATPase function, kinetoplast dependency, and drug sensitivity in trypanosomes. Proc. Natl. Acad. Sci. USA 112, 9112–9117 (2015).
Yambire, K. F. et al. Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. Elife 8, e51031 (2019).
Hughes, A. L. & Gottschling, D. E. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature 492, 261–265 (2012).
Chondrogianni, N. & Gonos, E. S. Proteasome dysfunction in mammalian aging: steps and factors involved. Exp. Gerontol. 40, 931–938 (2005).
Ruano, D. Proteostasis dysfunction in aged mammalian cells. The stressful role of inflammation. Front. Mol. Biosci. 8, 658742 (2021).
Sun-Wang, J. L., Ivanova, S. & Zorzano, A. The dialogue between the ubiquitin-proteasome system and autophagy: Implications in ageing. Ageing Res. Rev. 64, 101203 (2020).
Meller, A. & Shalgi, R. The aging proteostasis decline: from nematode to human. Exp. Cell Res. 399, 112474 (2021).
Guerrero-Navarro, L., Jansen-Durr P. & Cavinato M. Age-related lysosomal dysfunctions. Cells 11, 1–20 (2022).
Li, G. et al. Downregulation of NEDD4L by EGFR signaling promotes the development of lung adenocarcinoma. J. Transl. Med. 20, 47 (2022).
Martinez-Zamudio, R. I. et al. AP-1 imprints a reversible transcriptional programme of senescent cells. Nat. Cell Biol. 22, 842–855 (2020).
Giri, H. et al. Thrombomodulin regulation of mitogen-activated protein kinases. Int. J. Mol. Sci. 20, 1851 (2019).
Koizume, S. & Miyagi, Y. Diverse mechanisms of Sp1-dependent transcriptional regulation potentially involved in the adaptive response of cancer cells to oxygen-deficient conditions. Cancers (Basel) 8, 2 (2015).
Vierbuchen, T. et al. AP-1 transcription factors and the BAF complex mediate signal-dependent enhancer selection. Mol. Cell 68, 1067–1082.e12 (2017).
Cui, A., Ding, D. & Li, Y. Regulation of hepatic metabolism and cell growth by the ATF/CREB family of transcription factors. Diabetes 70, 653–664 (2021).
Giri, H. et al. Thrombomodulin is essential for maintaining quiescence in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 118, e2022248118 (2021).
Bae, J. S., Yang, L. & Rezaie, A. R. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc. Natl. Acad. Sci. USA 104, 2867–2872 (2007).
Schuepbach, R. A. et al. Protease-activated receptor-1 cleaved at R46 mediates cytoprotective effects. J. Thromb. Haemost. 10, 1675–1684 (2012).
Antoniak, S. et al. Protease-activated receptor 1 activation enhances doxorubicin-induced cardiotoxicity. J. Mol. Cell Cardiol. 122, 80–87 (2018).
Grimsey, N. J. & Trejo, J. Integration of endothelial protease-activated receptor-1 inflammatory signaling by ubiquitin. Curr. Opin. Hematol. 23, 274–279 (2016).
Sinha, R. K. et al. PAR1 biased signaling is required for activated protein C in vivo benefits in sepsis and stroke. Blood 131, 1163–1171 (2018).
De Ceunynck, K. et al. PAR1 agonists stimulate APC-like endothelial cytoprotection and confer resistance to thromboinflammatory injury. Proc. Natl. Acad. Sci. USA 115, E982–E991 (2018).
Molinar-Inglis, O. et al. aPC/PAR1 confers endothelial anti-apoptotic activity via a discrete, beta-arrestin-2-mediated SphK1-S1PR1-Akt signaling axis. Proc. Natl. Acad. Sci. USA 118, e2106623118 (2021).
Arif, S. A. et al. Vorapaxar for reduction of thrombotic cardiovascular events in myocardial infarction and peripheral artery disease. Am. J. Health Syst. Pharm. 72, 1615–1622 (2015).
Arsenault, K. A. et al. Direct thrombin inhibitors in cardiovascular disease. Nat. Rev. Cardiol. 9, 402–414 (2012).
Smith, A., Baumgartner, K. & Bositis, C. Cirrhosis: Diagnosis and management. Am. Fam. Physician 100, 759–770 (2019).
Wang, F. D., Zhou, J. & Chen, E. Q. Molecular mechanisms and potential new therapeutic drugs for liver fibrosis. Front. Pharmacol. 13, 787748 (2022).
Poole, L. G. et al. Liver fibrosis is driven by protease-activated receptor-1 expressed by hepatic stellate cells in experimental chronic liver injury. Res. Pract. Thromb. Haemost. 4, 906–917 (2020).
Passman, A. M. et al. A modified choline-deficient, ethionine-supplemented diet reduces morbidity and retains a liver progenitor cell response in mice. Dis. Model Mech. 8, 1635–1641 (2015).
Du, K. et al. Inhibiting xCT/SLC7A11 induces ferroptosis of myofibroblastic hepatic stellate cells but exacerbates chronic liver injury. Liver Int. 41, 2214–2227 (2021).
Christian, L. S. et al. Resident memory T cells in tumor-distant tissues fortify against metastasis formation. Cell Rep. 35, 109118 (2021).
Chen, S. et al. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Yu, G. et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Gustavsson, E. K. et al. ggtranscript: an R package for the visualization and interpretation of transcript isoforms using ggplot2. Bioinformatics 38, 3844–3846 (2022).
Maeso-Diaz, R. et al. Aging reduces liver resiliency by dysregulating Hedgehog signaling. Aging Cell 21, e13530 (2022).
Acknowledgements
We thank Dr. Tso-Pan Yao and Dr. Nina Tsvetanova for their valuable insight and discussion on endosomal dynamics and signaling. We also thank Drs. Rui Chen, Handan Xiang, Yi Ding, and Omar Lopez for their extensive discussion and valuable input on the senescent field.
Funding
This work was supported by grants from National Institutes of Health (R01CA244564 to X.F.W., R01DK077794 to A.M.D.), Duke Eye Center EM Facility Core (P30EY005722 to V.Y.A.), NIH Pathway to Independence Award (K99EY033763 to T.R.L.), Florence McAlister Professorship of Medicine (A.M.D.).
Author information
Authors and Affiliations
Contributions
C.C.P., P.B.A., and X.F.W. conceived the study. C.C.P., R.M.D., T.R.L., L.T., Y.L., L.W., F.Y., T.Y., C.W., K.D., D.H., S.H.O., X.Y., V.Y.A., Q.J.L., and X.F.W. contributed to the methodology. C.C.P., R.M.D., T.R.L., K.X., L.T., Y.L., L.W., F.Y., T.Y., C.W., K.D., D.H., S.H.O., E.W., B.L., and M.C. performed the experiments and analyzed the data. X.F.W. and A.M.D. acquired the funding. X.F.W. supervised the study. C.C.P., P.B.A., and X.F.W. drafted the manuscript. C.C.P., R.M.D., K.D., T.R.L., P.B.A., and X.F.W. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, C.C., Maeso-Díaz, R., Lewis, T.R. et al. Antagonizing the irreversible thrombomodulin-initiated proteolytic signaling alleviates age-related liver fibrosis via senescent cell killing. Cell Res 33, 516–532 (2023). https://doi.org/10.1038/s41422-023-00820-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41422-023-00820-4
This article is cited by
-
Senolysis through thrombomodulation
Cell Research (2023)