Abstract
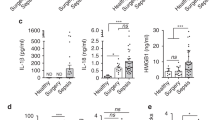
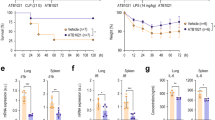
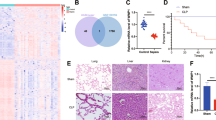
The NLRP3 inflammasome functions as an inflammatory driver, but its relationship with lipid metabolic changes in early sepsis remains unclear. Here, we found that GITR expression in monocytes/macrophages was induced by lysophosphatidylcholine (LPC) and was positively correlated with the severity of sepsis. GITR is a costimulatory molecule that is mainly expressed on T cells, but its function in macrophages is largely unknown. Our in vitro data showed that GITR enhanced LPC uptake by macrophages and specifically enhanced NLRP3 inflammasome-mediated macrophage pyroptosis. Furthermore, in vivo studies using either cecal ligation and puncture (CLP) or LPS-induced sepsis models demonstrated that LPC exacerbated sepsis severity/lethality, while conditional knockout of GITR in myeloid cells or NLRP3/caspase-1/IL-1β deficiency attenuated sepsis severity/lethality. Mechanistically, GITR specifically enhanced inflammasome activation by regulating the posttranslational modification (PTM) of NLRP3. GITR competes with NLRP3 for binding to the E3 ligase MARCH7 and recruits MARCH7 to induce deacetylase SIRT2 degradation, leading to decreasing ubiquitination but increasing acetylation of NLRP3. Overall, these findings revealed a novel role of macrophage-derived GITR in regulating the PTM of NLRP3 and systemic inflammatory injury, suggesting that GITR may be a potential therapeutic target for sepsis and other inflammatory diseases.

GITR exacerbates LPC-induced macrophage pyroptosis in sepsis via posttranslational regulation of NLRP3. According to the model, LPC levels increase during the early stage of sepsis, inducing GITR expression on macrophages. GITR not only competes with NLRP3 for binding to the E3 ligase MARCH7 but also recruits MARCH7 to induce the degradation of the deacetylase SIRT2, leading to decreasing ubiquitination but increasing acetylation of NLRP3 and therefore exacerbating LPC-induced NLRP3 inflammasome activation, macrophage pyroptosis and systemic inflammatory injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10.
van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407–20.
Samra JS, Summers LK, Frayn KN. Sepsis and fat metabolism. Br J Surg. 1996;83:1186–96.
Van Wyngene L, Vandewalle J, Libert C. Reprogramming of basic metabolic pathways in microbial sepsis: therapeutic targets at last? EMBO Mol Med. 2018;10:8712.
Rathinam VA, Fitzgerald KA. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165:792–800.
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588–606.
Wang L, Hauenstein AV. The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol Asp Med. 2020;76:100889.
Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89.
Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell. 2017;68:185–97.
Niu T, De Rosny C, Chautard S, Rey A, Patoli D, Groslambert M, et al. NLRP3 phosphorylation in its LRR domain critically regulates inflammasome assembly. Nat Commun. 2021;12:5862.
He M, Chiang HH, Luo H, Zheng Z, Qiao Q, Wang L, et al. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metab. 2020;31:580–91.
McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, et al. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23.
Shevach EM, Stephens GL. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6:613–8.
Santucci L, Agostini M, Bruscoli S, Mencarelli A, Ronchetti S, Ayroldi E, et al. GITR modulates innate and adaptive mucosal immunity during the development of experimental colitis in mice. Gut. 2007;56:52–60.
Xiao X, Shi X, Fan Y, Zhang X, Wu M, Lan P, et al. GITR subverts Foxp3+ Tregs to boost Th9 immunity through regulation of histone acetylation. Nat Commun. 2015;6:8266.
Wang B, Zhang W, Jankovic V, Golubov J, Poon P, Oswald EM, et al. Combination cancer immunotherapy targeting PD-1 and GITR can rescue CD8+ T cell dysfunction and maintain memory phenotype. Sci Immunol. 2018;3:7061.
Sabharwal SS, Rosen DB, Grein J, Tedesco D, Joyce-Shaikh B, Ueda R, et al. GITR agonism enhances cellular metabolism to support CD8+ T-cell proliferation and effector cytokine production in a mouse tumor model. Cancer Immunol Res. 2018;6:1199–211.
Shami A, Atzler D, Bosmans LA, Winkels H, Meiler S, Lacy M, et al. Glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) drives atherosclerosis in mice and is associated with an unstable plaque phenotype and cerebrovascular events in humans. Eur Heart J. 2020;41:2938–48.
Mu X, Wang P, Wang X, Li Y, Zhao H, Li Q, et al. Identification of a novel antisepsis pathway: sectm1a enhances macrophage phagocytosis of bacteria through activating GITR. J Immunol. 2020;205:1633–43.
Law SH, Chan ML, Marathe GK, Parveen F, Chen CH, Ke LY, An updated review of lysophosphatidylcholine metabolism in human diseases. Int J Mol Sci 2019;20:1149.
Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73.
Hao H, Cao L, Jiang C, Che Y, Zhang S, Takahashi S, et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab. 2017;25:856–67.
Martínez-García JJ, Martínez-Banaclocha H, Angosto-Bazarra D, de Torre-Minguela C, Baroja-Mazo A, Alarcón-Vila C, et al. P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis. Nat Commun. 2019;10:2711.
Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer. 2016;67:1–10.
Zhu K, Baudhuin LM, Hong G, Williams FS, Cristina KL, Kabarowski JH, et al. Sphingosylphosphorylcholine and lysophosphatidylcholine are ligands for the G protein-coupled receptor GPR4. J Biol Chem. 2001;276:41325–35.
Kabarowski JH, Zhu K, Le LQ, Witte ON, Xu Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293:702–5.
Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, et al. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98.
Li R, Guan Z, Bi S, Wang F, He L, Niu X, et al. The proton-activated G protein-coupled receptor GPR4 regulates the development of osteoarthritis via modulating CXCL12/CXCR7 signaling. Cell Death Dis. 2022;13:152.
Scumpia PO, Delano MJ, Kelly-Scumpia KM, Weinstein JS, Wynn JL, Winfield RD, et al. Treatment with GITR agonistic antibody corrects adaptive immune dysfunction in sepsis. Blood. 2007;110:3673–81.
Song H, Liu B, Huai W, Yu Z, Wang W, Zhao J, et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat Commun. 2016;7:13727.
Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–8.
Wang D, Zhang Y, Xu X, Wu J, Peng Y, Li J, et al. YAP promotes the activation of NLRP3 inflammasome via blocking K27-linked polyubiquitination of NLRP3. Nat Commun. 2021;12:2674.
Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68.
Shi X, Wu W, Feng Z, Fan P, Shi R, Zhang X. MARCH7-mediated ubiquitination decreases the solubility of ATG14 to inhibit autophagy. Cell Rep. 2023;42:113045.
Shi X, Zhang X. Control of ATG14 solubility and autophagy by MARCHF7/MARCH7-mediated ubiquitination. Autophagy. 2024;20:699–700.
Narita T, Weinert BT, Choudhary C.Functions and mechanisms of non-histone protein acetylation. Nat Rev Mol Cell Biol. 2019;20:156-174.
Wang Y, Cao C, Zhu Y, Fan H, Liu Q, Liu Y, et al. TREM2/β-catenin attenuates NLRP3 inflammasome-mediated macrophage pyroptosis to promote bacterial clearance of pyogenic bacteria. Cell Death Dis. 2022;13:771.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31970893, 32270976), the Natural Science Foundation of Guangdong Province (2022A1515012541), the Guangdong Natural Science Fund for Distinguished Young Scholars (2016A030306004), the Fundamental Research Funds for the Central Universities (2023kypt18; 2023ptpy67; 19ykzd39; 19ykpy43), the China Postdoctoral Science Foundation (2022M723661), the 111 Project (No. B12003, B13037) and the Open Project of the Key Laboratory of Tropical Disease Control (Sun Yat-sen University), Ministry of Education (2020kfkt08).
Author information
Authors and Affiliations
Contributions
SL, JZ, CC, YL, SM, XL, YS, JL, QP and JY conducted the experiments. SL, JZ, and CC acquired the data. SL, YL, SM, and MW provided scientific expertise and reagents. SL and MW designed the studies, analyzed the data, and wrote the manuscript. All the authors read the final version of the manuscript and approved its submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, S., Zhou, J., Cao, C. et al. GITR exacerbates lysophosphatidylcholine-induced macrophage pyroptosis in sepsis via posttranslational regulation of NLRP3. Cell Mol Immunol (2024). https://doi.org/10.1038/s41423-024-01170-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41423-024-01170-w