Abstract
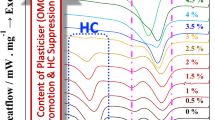
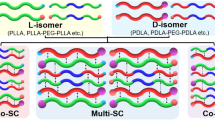
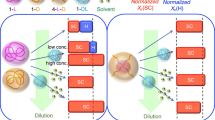
Polylactide (PLA) enantiomers of poly(l-lactide) (PLLA) and poly(d-lactide) (PDLA) were melt-blended with poly(methyl methacrylate) (PMMA) and compression-molded at a temperature between the melting points of PLA homocrystals (Tm,HC) and stereocomplex (SC) crystals (Tm,SC), causing the selective formation of SC crystallites. The degree of crystallinity of the SC crystals (χc,SC) did not change with the PMMA weight fraction but did vary with changes in the weight ratio of PLLA to PDLA. From differential scanning calorimetry (DSC) and dynamic mechanical analysis (DMA) measurements, the miscibility of PLA and PMMA was confirmed, but the formation of SC crystals during melt-blending induced phase separation into PLA-rich and PMMA-rich phases. The formation of homocrystals was hindered by increases in the weight fraction of PMMA and χc,SC. The thermal and viscoelastic properties of the PLLA/PDLA/PMMA blends were also affected by the PMMA weight fraction and χc,SC. According to the DMA results, the storage modulus of the ternary blends with higher χc,SC values showed a gentler decrease at the glass transition temperature; the ternary blends also exhibited a higher storage modulus than the PLLA/PMMA blends at high temperatures near Tm,HC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
De Santis P, Kovacs AJ. Molecular conformation of poly(s-lactic acid). Biopolymers. 1968;6:299–306.
Sasaki S, Asakura T. Helix distortion and crystal structure of the α-Form of poly(l-lactide). Macromolecules. 2003;36:8385–90.
Zhang J, Duan Y, Sato H, Tsuji H, Noda I, Yan S, et al. Crystal modifications and thermal behavior of poly(l-lactic acid) revealed by infrared spectroscopy. Macromolecules. 2005;38:8012–21.
Marubayashi H, Akaishi S, Akasaka S, Asai S, Sumita M. Crystalline structure and morphology of poly(l-lactide) formed under high-pressure CO2. Macromolecules. 2008;41:9192–203.
Eling B, Gogolewski S, Pennings AJ. Biodegradable materials of poly(l-lactic acid): 1. Melt-spun and solution-spun fibres. Polymer. 1982;23:1587–93.
Puiggali J, Ikada Y, Tsuji H, Cartier H, Okihara T, Lotz B. The frustrated structure of poly(l-lactide). Polymer. 2000;41:8921–31.
Cartier L, Okihara T, Ikada Y, Tsuji H, Puiggali J, Lotz B. Epitaxial crystallization and crystalline polymorphism of polylactides. Polymer. 2000;41:8909–19.
Okihara T, Tsuji M, Kawaguchi A, Katayama K, Tsuji H, Hyon S-H, et al. Crystal structure of stereocomplex of poly(L-lactide) and poly(D-lactide). J Macromol Sci Phys-B. 1991;30:119–40.
Tsuji H, Ikada Y. Crystallization from the melt of poly(lactide)s with different optical purities and their blends. Macromol Chem Phys. 1996;197:3483–99.
Shi X, Jing Z, Zhang G. Influence of PLA stereocomplex crystals and thermal treatment temperature on the rheology and crystallization behavior of asymmetric poly(L-lactide)/poly(D-lactide) blends. J Polym Res. 2018;25:71–86.
Wei XF, Bao RY, Cao ZQ, Yang W, Xie BH, Yang MB. Stereocomplex crystallite network in asymmetric PLLA/PDLA blends: formation, structure, and confining effect on the crystallization rate of homocrystallites. Macromolecules. 2014;47:1439–48.
Bao RY, Yang W, Jiang WR, Liu ZY, Xie BH, Yang MB, et al. Stereocomplex formation of high-molecular-weight polylactide: a low temperature approach. Polymer. 2012;53:5449–54.
Pan P, Han L, Bao J, Xie Q, Shan G, Bao Y. Competitive stereocomplexation, homocrystallization, and polymorphic crystalline transition in poly(l-lactic acid)/poly(d-lactic acid) racemic blends: molecular weight effects. J Phys Chem B. 2015;119:6462–70.
Tsuji H, Ikada Y. Stereocomplex formation between enantiomeric poly(lactic acids). XI. Mechanical properties and morphology of solution-cast films. Polymer. 1993;26:6918–26.
Bao RY, Yang W, Jiang WR, Liu ZY, Xie BH, Yang MB. Polymorphism of racemic poly(l-lactide)/poly(d-lactide) blend: effect of melt and cold crystallization. J Phys Chem B. 2013;117:3667–74.
López-Rodríguez N, Martínez de Arenaza I, Meaurio E, Sarasua JR. Efficient stereocomplex crystallization in enantiomeric blends of high molecular weight polylactides. ACS Adv 2015;44:34525–34.
Cedric S, Jean-Marie R, Philippe D. PLLA/PMMA blends: a shear-induced miscibility with tunable morphologies and properties? Polymer. 2013;54:3931–9.
Shirahase T, Komatsu Y, Tominaga Y, Asai S, Sumita M. Miscibility and hydrolytic degradation in alkaline solution of poly(L-lactide) and poly(methyl methacrylate) blends. Polymer. 2006;47:4839–44.
Hirota S, Sato T, Tominaga Y, Asai S, Sumita M. The effect of high-pressure carbon dioxide treatment on the crystallization behavior and mechanical properties of poly(L-lactic acid)/poly(methyl methacrylate) blends. Polymer. 2006;47:3954–60.
Samuel C, Cayuela J, Barakat I, Müller AJ, Raquez J-M, Dubois P. Stereocomplexation of polylactide enhanced by poly(methyl methacrylate): improved processability and thermomechanical properties of stereocomplexable polylactide-based materials. ACS Appl Mater Interfaces. 2013;5:11797–807.
Liu T, Xiang F, Qi X, Yang W, Huang R, Fu Q. Optically transparent poly(methyl methacrylate) with largely enhanced mechanical and shape memory properties via in-situ formation of polylactide stereocomplex in the matrix. Polymer. 2017;126:231–9.
Bao RY, Yang W, Liu ZY, Xie BH, Yang MB. Polymorphism of a high-molecular-weight racemic poly(L-lactide)/poly(D-lactide) blend: effect of melt blending with poly(methyl methacrylate). RSC Adv. 2015;5:19058–66.
Dong Q, Bian Y, Li Y, Han C, Dong L. Miscibility and crystallization behaviors of stereocomplex-type poly(L- and D-lactide)/poly(methyl methacrylate) blends. J Therm Anal Calor. 2014;118:359–67.
Tsuji H. Poly(lactide) stereocomplexes: formation, structure, properties, degradation, and applications. Macromol Biosci. 2005;5:569–97.
Tsuji H, Horii F, Nakagawa M, Ikada Y, Odani H, Kitamura R. Stereocomplex formation between enantiomeric poly(lactic acid)s. 7. Phase structure of the stereocomplex crystallized from a dilute acetonitrile solution as studied by high-resolution solid-state carbon-13 NMR spectroscopy. Macromolecules. 1992;25:4114–8.
Fischer EW, Sterzel HJ, Wegner GK. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z Z Polym. 1973;251:980–90.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iguchi, Y., Akasaka, S. & Asai, S. Formation of PLA stereocomplex crystals during melt-blending of asymmetric PLLA/PDLA/PMMA blends of varying miscibility. Polym J 52, 225–235 (2020). https://doi.org/10.1038/s41428-019-0256-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-019-0256-6