Abstract
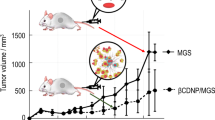
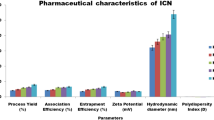
α-Mangostin (MGS), an anti-cancer compound, is a xanthone derivative and is extracted from the pericarps of mangosteen. MGS exhibits a variety of bioactivities, such as antioxidant, cytotoxic, anti-inflammatory, and antibacterial effects, as well as anticancer activity. However, MGS has not been approved for clinical use because of its poor bioavailability. There have been many efforts to solve this problem by use of drug carriers. Cyclodextrins (CDs) are well known as nontoxic and biodegradable drug carriers and can encapsulate MGS. In this study, we prepared CD-based nanoparticles (CDNPs) by a polyaddition reaction using epichlorohydrin and characterized them by dynamic light scattering and static light scattering coupled with fractionation. The encapsulation of MGS into CDNPs was examined, and we found that the loading ratio of MGS for CDNPs is much higher than that for CDs themselves. The cytotoxicity of the CDNP/MGS complex was examined, indicating the potential of CDNP as a carrier of MGS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Saurabh P, Paul NS, Amitha KH. Review of procedures used for the extraction of anti-cancer compounds from tropical plants. Anti-Cancer Agents Med Chem. 2015;15:314–26. https://doi.org/10.2174/1871520614666141114202104.
Loftsson T, Hreinsdóttir D, Másson M. The complexation efficiency. J Incl Phenom Macrocycl Chem. 2007;57:545–52.
Pinto MMM, Sousa ME, Nascimento MSJ. Xanthone derivatives: new insights in biological activities. Curr Med Chem. 2005;12:2517–38.
Chun-Nan L, Shiou-Jyh L, Tai-Hua L, Yin-Ching C, Shen-Jeu W. Xanthone derivatives as potential anti-cancer drugs. J Pharm Pharmacol. 1996;48:539–44.
Madalena P, Fátima C, Maria Emília S, Maria S, Jose’ N, Madalena P. Xanthones as inhibitors of growth of human cancer cell lines and their effects on the proliferation of human lymphocytes in vitro. Bioorg Med Chem. 2002;10:3725–30.
Na Y. Recent cancer drug development with xanthone structures. J Pharm Pharmacol. 2009;61:707–12.
Han AR, Kim JA, Lantvit DD, Kardono LB, Riswan S, Chai H, et al. Cytotoxic xanthone constituents of the stem bark of Garcinia mangostana (mangosteen). J Nat Prod. 2009;72:2028–31.
Hyun-Ah J, Bao-Ning S, William J, Rajendra K, G M, Douglas A, et al. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J Agric Food Chem. 2006;54:2077–82.
Pedraza-Chaverri J, Cardenas-Rodriguez N, Orozco-Ibarra M, Perez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem Toxicol. 2008;46:3227–39.
Keigo N, Norimichi N, Tsutomu A, Hideyuki Y, Yasushi O. Inhibiton of cyclooxygenase and prostaglandin E2 synthesis by y-MGS, a xanthone dervative in mangosteen, in C6 rat glioma cell. Biochem Pharmacol. 2002;63:73–79.
Janhom P, Dharmasaroja P. Neuroprotective effects of alpha-mangostin on MPP(+)-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. J Toxicol. 2015;2015:1–11.
Yukihiro A, Yoshihito N, Munekazu I, Yoshinori N. Anti-cancer effects of xanthones from pericarps of mangosteen. Int J Mol Sci. 2008;9:355–70.
U.S. Department of Health and Human Services. Drug Products, Including Biological Products, that Contain Nanomaterials Guidance for Industry. Pharmaceutical Quality/CMC. 2017. https://www.fda.gov/media/109910/download.
Pan-In P, Wongsomboon A, Kokpol C, Chaichanawongsaroj N, Wanichwecharungruang S. Depositing alpha-mangostin nanoparticles to sebaceous gland area for acne treatment. J Pharm Sci. 2015;129:226–32.
Yostawonkul J, Surassmo S, Namdee K, Khongkow M, Boonthum C, Pagseesing S, et al. Nanocarrier-mediated delivery of alpha-mangostin for non-surgical castration of male animals. Sci Rep. 2017;7:16234–41.
Mochizuki S, Sakurai K. A novel polysaccharide/polynucleotide complex and its application to bio-functional DNA delivery system. Polym J. 2009;41:343–53.
He Y, Fu P, Shen X, Gao H. Cyclodextrin-based aggregates and characterization by microscopy. Micron. 2008;39:495–516.
Gidwani B, Vyas A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed Res Int. 2015;2015:198–268.
Collins CJ, McCauliff LA, Hyun SH, Zhang Z, Paul LN, Kulkarni A, et al. Synthesis, characterization, and evaluation of pluronic-based beta-cyclodextrin polyrotaxanes for mobilization of accumulated cholesterol from Niemann-Pick type C fibroblasts. Biochemistry. 2013;52:3242–53.
Zhang J, Ma PX. Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv Drug Deliv Rev. 2013;65:1215–33.
Simoes SM, Rey-Rico A, Concheiro A, Alvarez-Lorenzo C. Supramolecular cyclodextrin-based drug nanocarriers. Chem Commun (Camb). 2015;51:6275–89.
Wei H, Yu CY. Cyclodextrin-functionalized polymers as drug carriers for cancer therapy. Biomater Sci. 2015;3:1050–60.
Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Formulation and biological evaluation of glimepiride-cyclodextrin-polymer systems. Int J Pharm. 2006;309:129–38.
Ozdemir N, Erkin J. Enhancement of dissolution rate and bioavailability of sulfamethoxazole by complexation with beta-cyclodextrin. Drug Dev Ind Pharm. 2012;38:331–40.
Rungnim C, Phunpee S, Kunaseth M, Namuangruk S, Rungsardthong K, Rungrotmongkol T, et al. Co-solvation effect on the binding mode of the alpha-mangostin/beta-cyclodextrin inclusion complex. Beilstein J Org Chem. 2015;11:2306–17.
Phunpee S, Suktham K, Surassmo S, Jarussophon S, Rungnim C, Soottitantawat A, et al. Controllable encapsulation of alpha-mangostin with quaternized beta-cyclodextrin grafted chitosan using high shear mixing. Int J Pharm. 2018;538:21–29.
Qin X, Bai L, Tan Y, Li L, Song F, Wang Y. β-Cyclodextrin-crosslinked polymeric adsorbent for simultaneous removal and stepwise recovery of organic dyes and heavy metal ions: fabrication, performance and mechanisms. Chem Eng J. 2019;372:1007–18.
Renard E, Deratani A, Volet G, Sebille B. Preparation and characterization of water soluble high molecular weight β-cyclodextrin-epichlorohydrin polymers. Eur Polym J. 1997;33:49–57.
Mariuca-Roxana G, Mihai N, Emma-Adriana B, Dumitru L, Corina A. Phase solubility studies and scanning electron microscopy of Dexamethasone inclusion complexes with β-cyclodextrin and hydroxypropyl β -cyclodextrin. Vasile Goldis Univ Press. 2012;22:83–93.
Heydari A, Hassani Y, Sheibani H, Pardakhti A. Water-soluble β-cyclodextrin polymers as drug carriers to improve solubility, thermal stability and controlled release of nifedipine. Pharm Chem J. 2017;51:375–83.
Salgın S, Salgın U, Ayluçtarhan M. Synthesis of β-cyclodextrin-epichlorohydrin nanospheres: its application for removal of p-nitrophenol. Am Chem Sci J. 2016;16:1–10.
Oswald ST, Alexei AF, Paul GL, Timothy AG. Binding of aliphatic ketones to cyclodextrins in quaeous solution. J Chem Soc, Perkin Trans. 1996;2:1243–49.
Saokham P, Muankaew C, Jansook P, Loftsson T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules. 2018;23:1161–76.
Yong CW, Washington C, Smith W. Structural behaviour of 2-hydroxypropyl-beta-cyclodextrin in water: molecular dynamics simulation studies. Pharm Res. 2008;25:1092–99.
Alsbaiee A, Smith BJ, Xiao L, Ling Y, Helbling DE, Dichtel WR. Rapid removal of organic micropollutants from water by a porous beta-cyclodextrin polymer. Nature. 2016;529:190–94.
Acknowledgements
This work was supported by JST CREST Grant Number JPMJCR1521, Japan. This work was also supported by a JSPS Grant-in-Aid for Scientific Research (B) (Grant Number 17K14073).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Doan, V.T.H., Lee, J.H., Takahashi, R. et al. Cyclodextrin-based nanoparticles encapsulating α-mangostin and their drug release behavior: potential carriers of α-mangostin for cancer therapy. Polym J 52, 457–466 (2020). https://doi.org/10.1038/s41428-019-0296-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-019-0296-y
This article is cited by
-
Determining the critical quality attribute for the delivery of α–mangostin by β–cyclodextrin-based nanoparticles in cancer treatment
Polymer Journal (2023)
-
Characteristics and Bioactivities of Carrageenan/Chitosan Microparticles Loading α-Mangostin
Journal of Polymers and the Environment (2022)
-
Evaluation of the supramolecular structure of drug delivery carriers using synchrotron X-ray scattering
Polymer Journal (2021)
-
Anticancer efficacy of cyclodextrin-based hyperbranched polymer nanoparticles containing alpha-mangostin
Polymer Journal (2021)
-
Application of nanomaterials decorated with cyclodextrins as sensing elements for environment analysis
Environmental Science and Pollution Research (2021)