Abstract
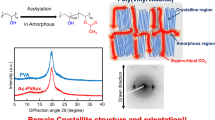
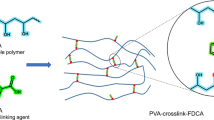
Poly(vinyl butyral) (PVB) is widely accepted as an adhesive for glass substrates within the automobile windows. In this work, we suggested and performed butyralization of poly(vinyl alcohol) (PVA) under supercritical carbon dioxide (sc-CO2) and investigated not only the structure and mechanical properties of the obtained PVB but also its adhesion properties under various conditions, comparing this PVA with other PVBs prepared in the solution and swollen states. The conversion ratio of butyralization under sc-CO2 was larger than that in the swollen state and lower than that in the solution state and exhibited a sufficient material performance as the adhesive for glass substrates. The Young’s modulus and tensile strength of PVB under sc-CO2 were higher than those of the other PVBs. The mechanical properties of the PVB prepared under sc-CO2 have no correlation to the modification ratios because sc-CO2 penetrated into the amorphous region of the PVA and preferentially modified its hydroxyl groups. Furthermore, the adhesive strengths of all the obtained PVBs increased, and under a high-humidity atmosphere, the adhesive strength of the PVB prepared under sc-CO2 was the largest. The humidity resistance of the PVB adhesive prepared under sc-CO2 was proven.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Delattre E, Lemière G, Desmurs J-R, Boulay B, Duñach E. Poly(vinyl alcohol) functionalization with aldehydes in organic solvents: shining properties of poly(vinyl acetals). J Appl Polym Sci. 2014;131:40677.
Giménez V, Mantecón A, Cádiz V. Modification of poly(vinyl alcohol) with acid chlorides and crosslinking with difunctional hardeners. J Polym Sci Part A Polym Chem. 1996;34:925–34.
Flory PJ. Intramolecular reaction between neighboring substituents of Vinylpolymers. J Am Chem Soc. 1939;61:1518–21.
Gaina C, Gaina V, Ionita D. Functional modification of poly(vinyl alcohol) with maleimide compounds. Polym Bull. 2016;73:2019–38.
Zhao B, Lu CH, Liang M. Solvent-free esterification of poly(vinyl alcohol) and maleic anhydride through mechanochemical reaction. Chin Chem Lett. 2007;18:1353–6.
Anbarasan R, Pandiarajaguru R, Prabhu R, Dhanalakshmi V, Jayalakshmi A, Dhanalakshmi B, et al. Synthesis, characterizations, and mechanical properties of structurally modified poly(vinyl alcohol). J Appl Polym Sci. 2010;117:2059–68.
Gaina C, Ursache O, Gaina V, Ionita D. Study on the chemical modification of Poly(Vinyl Alcohol) with 4-maleimidophenyl isocyanate. Polym Plast Technol Eng. 2012;51:65–70.
Sakurada I. 10. Formalization in Polyvinyl Alcohol Fibers. Marcel Dekker, Inc.; 1985. p. 211–30. ISBN:0-8247-7434-5.
Nguyen FN, Berg JC. The effect of vinyl alcohol content on adhesion performance in poly(vinyl butyral)/glass systems. J Adhes Sci Technol. 2004;18:1011–26.
Chen W, David DJ, MacKnight WJ, Karasz FE. Miscibility and phase behavior in blends of Poly(vinyl butyral) and poly(methyl methacrylate). Macromolecules. 2001;34:4277–84.
Elzière P, Fourton P, Demassieux Q, Chennevière A, Dalle-Ferrier C, Creton C, et al. Supramolecular structure for large strain dissipation and outstanding impact resistance in polyvinylbutyral. Macromolecules. 2019;52:7821–30.
Huntsberger JR. Adhesion of Plasticized Polyvinyl Butyral) to Glass. J Adhes. 1981;13:107–29.
Zhu G, Cui X, Zhang Y, Chen S, Dong M, Liu H, et al. Poly (vinyl butyral)/Graphene oxide/poly (methylhydrosiloxane) nanocomposite coating for improved aluminum alloy anticorrosion. Polymer. 2019;172:415–22.
Tsugawa N, Ito A, Yamaguchi M. Effect of lithium salt addition on the structure and optical properties of PMMA/PVB blends. Polymer. 2018;146:242–8.
Pizzanelli S, Prevosto D, Labardi M, Guazzini T, Bronco S, Forte C, et al. Dynamics of poly(vinyl butyral) studied using dielectric spectroscopy and 1H NMR relaxometry. Phys Chem Chem Phys. 2017;19:31804–12.
Yang B, Liu R, Huang J, Sun H. Reverse dissolution as a route in the synthesis of poly(vinyl butyral) with high butyral contents. Ind Eng Chem Res 2013;52:7425–31.
Dasgupta AM, David DJ, Misra A. Synthesis and characterization of ionomeric poly(vinyl butyral). J Appl Polym Sci 1992;44:1213–21.
Fernández MD, Fernández MJ, Hoces P. Synthesis of poly(vinyl butyral)s in homogeneous phase and their thermal properties. J Appl Polym Sci. 2006;102:5007–17.
Saravanan S, Akshay Gowda KM, Arul Varman K, Ramamurthy PC, Madras G. In-situ synthesized poly(vinyl butyral)/MMT-clay nanocomposites: The role of degree of acetalization and clay content on thermal, mechanical and permeability properties of PVB matrix. Compos Sci Technol. 2015;117:417–27.
Chetri P, Dass NN. Preparation of poly(vinyl butyral) with high acetalization rate. J Appl Polym Sci. 2001;81:1182–6.
Chang BH, Zeigler R, Hiltner A. Chlorinated high density polyethylene. I. Chain characterization. Polym Eng Sci. 1988;28:1167–72.
Jung J, Perrut M. Particle design using supercritical fluids: Literature and patent survey. J Supercrit Fluids. 2001;20:179–219.
Yeo S-D, Kiran E. Formation of polymer particles with supercritical fluids: a review. J Supercrit Fluids. 2005;34:287–308.
Koytsoumpa EI, Bergins C, Kakaras E. The CO2 economy: review of CO2 capture and reuse technologies. J Supercrit Fluids. 2018;132:3–16.
Meziani MJ, Pathak P, Desai T, Sun Y-P. Supercritical fluid processing of nanoscale particles from biodegradable and biocompatible polymers. Ind Eng Chem Res. 2006;45:3420–4.
Lee H, Terry E, Zong M, Arrowsmith N, Perrier S, Thurecht KJ, et al. Successful dispersion polymerization in supercritical CO2 using polyvinylalkylate hydrocarbon surfactants synthesized and anchored via RAFT. J Am Chem Soc 2008;130:12242–3.
Ivanovic J, Milovanovic S, Zizovic I. Utilization of supercritical CO2 as a processing aid in setting functionality of starch-based materials. Starch - Stärke. 2016;68:821–33.
Yalpani M. Supercritical fluids: puissant media for the modification of polymers and biopolymers. Polymer. 1993;34:1102–5.
Nishino T, Kotera M, Suetsugu M, Murakami H, Urushihara Y. Acetylation of plant cellulose fiber in supercritical carbon dioxide. Polymer. 2011;52:830–6.
Owens DK, Wendt RC. Estimation of the surface free energy of polymers. J Appl Polym Sci. 1969;13:1741–7.
Fowkes FM. ATTRACTIVE FORCES AT INTERFACES. Ind Eng Chem. 1964;56:40–52.
Bruch MD, Bonesteel JAK. Interpretation of the proton NMR spectrum of poly(vinyl butyral) by two-dimensional NMR. Macromolecules. 1986;19:1622–7.
Liau LCK, Yang TCK, Viswanath DS. Mechanism of degradation of poly(vinyl butyral) using thermogravimetry/fourier transform infrared spectrometry. Polym Eng Sci. 1996;36:2589–2600.
Prosanov IY, Matvienko AA. Study of PVA thermal destruction by means of IR and Raman spectroscopy. Phys Solid State. 2010;52:2203–6.
Fahs GB, Benson SD, Moore RB. Blocky sulfonation of syndiotactic polystyrene: a facile route toward tailored ionomer architecture via postpolymerization functionalization in the gel state. Macromolecules. 2017;50:2387–96.
Noble KF, Noble AM, Talley SJ, Moore RB. Blocky bromination of syndiotactic polystyrene via post-polymerization functionalization in the heterogeneous gel state. Polym Chem. 2018;9:5095–106.
Anderson LJ, Yuan X, Fahs GB, Moore RB. Blocky Ionomers via Sulfonation of Poly(ether ether ketone) in the Semicrystalline Gel State. Macromolecules. 2018;51:6226–37.
Anderson LJ, Moore RB. Sulfonation of blocky brominated PEEK to prepare hydrophilic-hydrophobic blocky copolymers for efficient proton conduction. Solid State Ion. 2019;336:47–56.
Parker AA, Hedrick DP, Ritchey WM. Studies of thermal transition behavior in plasticized poly(vinyl butyral-co-vinyl alcohol) with solid-state NMR and thermal analysis techniques. J Appl Polym Sci. 1992;46:295–301.
Dhaliwal AK, Hay JN. The characterization of polyvinyl butyral by thermal analysis. Thermochim Acta. 2002;391:245–55.
Carini G Jr., Carini G, D’Angelo G, Federico M, Di Marco G, Bartolotta A. Enhancing the molecular cooperativity of polyvinyl butyral using liquid additives. J Polym Sci Part B Polym Phys. 2018;56:340–6.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP12345678.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Matsumoto, T., Yorifuji, M., Sugiyama, Y. et al. Butyralization of poly(vinyl alcohol) under supercritical carbon dioxide for a humidity-resistant adhesive to glass substrates. Polym J 52, 1349–1356 (2020). https://doi.org/10.1038/s41428-020-00402-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-00402-w