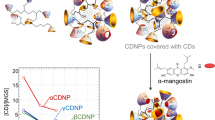
Abstract
Alpha-mangostin (MGS) is a natural xanthone compound extracted from mangosteen pericarps. It has great potential as an excellent anticancer agent. However, the extremely low solubility of MGS in water seriously impedes its medical application. Previously, we found that cyclodextrin (CD)-based hyperbranched polymer nanoparticles (CDNPs) solubilize MGS by encapsulating it in the CD cavity and that their binding constants are 100 times higher than those of native CDs. Our findings suggested that CDNPs could be good carriers of MGS. Here, we prepared three types of CDNP from α-, β-, and ɣCDs and compared them in terms of MGS release and in vitro and in vivo anticancer efficacy. βCDNP/MGS demonstrated the greatest anticancer efficacy, while no efficacy was observed for the other CDNPs. MGS release from CDNPs/MGS can be explained by a model in which the slow and rapid modes are connected in series; before release, MGS must shift from the slow to the rapid mode. We assumed that the slow and rapid modes are related to the interior and surface CDs of CDNPs. βCDNP/MGS showed the slowest release in the slow mode. We assume that slow release in the slow mode is essential for MGS retention until the cancerous region is reached.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Phan G, Hideaki O. New compounds and potential candidates for drug discovery from medicinal plants of Vietnam. Chem Pharm Bull. 2018;66:493.
Janhom P, Dharmasaroja P. Neuroprotective effects of alpha-mangostin on MPP(+)-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. J Toxicol. 2015;2015:1–11. https://doi.org/10.1155/2015/919058.
Jung H-A, Su B-N, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J Agric Food Chem. 2006;54:2077–82.
Pedraza-Chaverri J, Cardenas-Rodriguez N, Orozco-Ibarra M, Perez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem Toxicol. 2008;46:3227–39. https://doi.org/10.1016/j.fct.2008.07.024.
Keigo N, Norimichi N, Tsutomu A, Hideyuki Y, Yasushi O. Inhibiton of cyclooxygenase and prostaglandin E2 synthesis by ɣ-MGS, a xanthone dervative in mangosteen, in C6 rat glioma cell. Biochem Pharm. 2002;63:73–79.
Akao Y, Nakagawa Y, Iinuma M, Nozawa Y. Anti-cancer effects of xanthones from pericarps of mangosteen. Inter J Mol Sci. 2008;9:355–70.
Tokunaga E, Oki E, Egashira A, Sadanaga N, Morita M, Kakeji Y, Maehara Y. Deregulation of the Akt pathaway in human cancer. Curr Cancer Drug Targets. 2008;8:27–36.
National Center for Biotechnology Information. PubChem Database. alpha-Mangostin, https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Mangostin.
Rungnim C, Phunpee S, Kunaseth M, Namuangruk S, Rungsardthong K, Rungrotmongkol T, Ruktanonchai U. Co-solvation effect on the binding mode of the alpha-mangostin/beta-cyclodextrin inclusion complex. Beilstein J Org Chem. 2015;11:2306–17. https://doi.org/10.3762/bjoc.11.251.
Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–9. https://doi.org/10.1016/j.addr.2012.10.002.
Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjugate Chem. 2010;21:797–802.
Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–23. https://doi.org/10.1038/nnano.2011.166.
Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252–76. https://doi.org/10.1016/j.msec.2019.01.066.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Rel. 2000;65:271–84.
Maeda H, Tsukigawa K, Fang J. A retrospective 30 years after discovery of the enhanced permeability and retention effect of solid tumors: next-generation chemotherapeutics and photodynamic therapy-problems, solutions, and prospects. Microcirculation. 2016;23:173–82. https://doi.org/10.1111/micc.12228.
Nakamura H, Etrych T, Chytil P, Ohkubo M, Fang J, Ulbrich K, Maeda H. Two step mechanisms of tumor selective delivery of N-(2-hydroxypropyl)methacrylamide copolymer conjugated with pirarubicin via an acid-cleavable linkage. J Control Rel. 2014;174:81–7. https://doi.org/10.1016/j.jconrel.2013.11.011.
Nakamura H, Koziolova E, Chytil P, Etrych T, Haratake M, Maeda H. Superior penetration and cytotoxicity of HPMA copolymer conjugates of pirarubicin in tumor cell spheroid. Mol Pharm. 2019;16:3452–59. https://doi.org/10.1021/acs.molpharmaceut.9b00248.
Wathoni N, Rusdin A, Motoyama K, Joni IM, Lesmana R, Muchtaridi M. Nanoparticle drug delivery systems for alpha-Mangostin. Nanotechnol Sci Appl. 2020;13:23–36. https://doi.org/10.2147/NSA.S243017.
Verma RK, Yu W, Shrivastava A, Shankar S, Srivastava RK. alpha-Mangostin-encapsulated PLGA nanoparticles inhibit pancreatic carcinogenesis by targeting cancer stem cells in human, and transgenic (Kras(G12D), and Kras(G12D)/tp53R270H) mice. Sci Rep. 2016;6:32743. https://doi.org/10.1038/srep32743.
Yostawonkul J, Surassmo S, Namdee K, Khongkow M, Boonthum C, Pagseesing S, Saengkrit N, Ruktanonchai UR, Chatdarong K, Ponglowhapan S, Yata T. Nanocarrier-mediated delivery of alpha-mangostin for non-surgical castration of male animals. Sci Rep. 2017;7:16234–41. https://doi.org/10.1038/s41598-017-16563-3.
Doan THV, Lee JH, Takahashi R, Nguyen TMP, Nguyen TVA, Pham TTH, Fujii S, Sakurai K. Cyclodextrin-based nanoparticles encapsulating α-mangostin and their drug release behavior: potential carriers of α-mangostin for cancer therapy. Polym J. 2020;52:457–66. https://doi.org/10.1038/s41428-019-0296-y.
Alsbaiee A, Smith BJ, Xiao L, Ling Y, Helbling DE, Dichtel WR. Rapid removal of organic micropollutants from water by a porous beta-cyclodextrin polymer. Nature 2016;529:190–94. https://doi.org/10.1038/nature16185.
Doan THV, Doan TNA, Fujii S, Sakurai K. Enhanced binding constant of cyclodextrin to alpha-mangostin in hyperbranched polymers. Chem Lett. 2020. https://doi.org/10.1246/cl.200210.
Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Disco. 2004;3:1023–35. https://doi.org/10.1038/nrd1576.
Suzie HP, Frederik T, Nathalie CB, Jianjun C, Brendan HG, Gregory SJ, Mark ED, Marcus B, Michel J, Boudewijn J, Wim F, Annette B. Targeted delivery of RNA-cleaving DNA enzyme (DNAzyme) to tumor tissue by transferrin-modified, cyclodextrin-based particles. Cancer Biol Ther. 2004;3:641–50. https://doi.org/10.4161/cbt.3.7.918.
Trotta F, Zanetti M, Cavalli R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J Org Chem. 2012;8:2091–9. https://doi.org/10.3762/bjoc.8.235.
D’Mello SR, Cruz CN, Chen M-L, Kapoor M, Lee SL, Tyner KM. The evolving landscape of drug products containing nanomaterials in the United States. Nat Nanotechnol. 2017;12:523–29. https://doi.org/10.1038/nnano.2017.67.
Caputo F, Clogston J, Calzolai L, Rosslein M, Prina-Mello A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J Control Rel. 2019;299:31–43. https://doi.org/10.1016/j.jconrel.2019.02.030.
Hotarat W, Phunpee S, Rungnim C, Wolschann P, Kungwan N, Ruktanonchai U, Rungrotmongkol T, Hannongbua S. Encapsulation of alpha-mangostin and hydrophilic beta-cyclodextrins revealed by all-atom molecular dynamics simulations. J Mol Liq. 2019;288:110965–73. https://doi.org/10.1016/j.molliq.2019.110965.
Lin J, Yu Y, Shigdar S, Fang DZ, Du JR, Wei MQ, Danks A, Liu K, Duan W. Enhanced antitumor efficacy and reduced systemic toxicity of sulfatide-containing nanoliposomal doxorubicin in a xenograft model of colorectal cancer. PLoS One. 2012;7:e49277. https://doi.org/10.1371/journal.pone.0049277.
Costa EC, Moreira AF, de Melo-Diogo D, Gaspar VM, Carvalho MP, Correia IJ. 3D tumor spheroids: an overview on the tools and techniques used for their analysis. Biotechnol Adv. 2016;34:1427–41. https://doi.org/10.1016/j.biotechadv.2016.11.002.
Li L, Brunner I, Han A, Hamburger M, Kinghorn AD, Frye R, Butterweck V. Pharmacokinetics of a-mangostin in rats after intravenous and oral application. Mol Nutr Food Res. 2011;55:567–74. https://doi.org/10.1002/mnfr.201000511.
Johnson JJ, Petiwala SM, Syed DN, Rasmussen JT, Adhami VM, Siddiqui IA, Kohl AM, Mukhtar H. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis. 2012;33:413–19. https://doi.org/10.1093/carcin/bgr291.
Lee HN, Jang HY, Kim HJ, Shin SA, Choo GS, Park YS, Kim SK, Jung JY. Antitumor and apoptosis-inducing effects of alpha-mangostin extracted from the pericarp of the mangosteen fruit (Garcinia mangostana L.) in YD-15 tongue mucoepidermoid carcinoma cells. Int J Mol Med. 2016;37:939–48. https://doi.org/10.3892/ijmm.2016.2517.
Shibata M, Iinuma M, Morimoto J, Kurose H, Akamatsu K, Okuno Y, Akao Y, Otsuki Y. α-Mangostin extracted from the pericarp of the mangosteen (Garcinia mangostana Linn) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation. BMC Med. 2011;9:69–87. http://www.biomedcentral.com/1741-7015/9/69.
Acknowledgements
This work was supported by JSPS KAKENHI: Grant-in-Aid for Scientific Research (Grant No. 19K15394), Grant-in-Aid for Scientific Research A (20H00668), and Grant-in-Aid for Challenging Exploratory Research (20K20449).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Doan, V.T.H., Takano, S., Doan, N.A.T. et al. Anticancer efficacy of cyclodextrin-based hyperbranched polymer nanoparticles containing alpha-mangostin. Polym J 53, 481–492 (2021). https://doi.org/10.1038/s41428-020-00441-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-00441-3