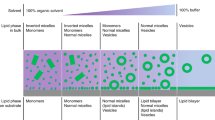
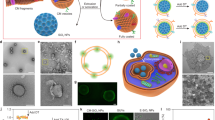
Abstract
Biological membranes that tailor their morphology to environmental stimuli are nature’s ultimate smart molecular systems. This focused review describes the design and function of smart biomembrane nanohybrids. Specifically, liposomes and exosomes can be functionalized with inorganic substances and polymers to afford hybridized artificial cell membranes. These membranes have a tailorable morphology and can incorporate functional integral membrane proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sasaki Y, Matsui K, Aoyama Y, Kikuchi J. Cerasome as an infusible and cell-friendly gene carrier: synthesis of cerasome-forming lipids and transfection using cerasome. Nat Protoc. 2006;1:1227–34.
Vargas K, Shon Y. Hybrid lipid–nanoparticle complexes for biomedical applications. J Mater Chem B. 2019;7:695–708.
Mohammadi M, Taghavi S, Abnos K, Taghdisi SM, Ramezani M, Alibolandi M. Hybrid vesicular drug delivery systems for cancer therapeutics. Adv Funct Mater. 2018;28:1802136.
Sasaki Y, Akiyoshi K. Nanogel engineering for new nanobiomaterials: from chaperoning engineering to biomedical applications. Chem Rec. 2010;10:366–76.
Sasaki Y, Akiyoshi K. Self-assembled nanogel engineering for advanced biomedical technology. Chem Lett. 2012;41:202–8.
Sekine Y, Moritani Y, Ikeda-Fukazawa T, Sasaki Y, Akiyoshi K. A hybrid hydrogel biomaterial by nanogel engineering: bottom-up design with nanogel and liposome building blocks to develop a multidrug delivery system. Adv Healthc Mater. 2012;1:722–8.
Ternullo S, Werning L, Holsater A, Basnet N. Curcumin-in-deformable liposomes-in-chitosan-hydrogel as a novel wound dressing. Pharmaceutics. 2020;12:8.
Xu S, An X. Preparation, microstructure and function for injectable liposome-hydrogels. Colloids Surf A Physicochem Eng Asp. 2019;560:20–5.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. https://doi.org/10.1126/science.aau6977.
Sato K, Umezaki K, Sawada S, Mukai S, Sasaki Y, Harada N, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933.
Akbari A, Jabbari N, Sharifi R, Ahmadi M, Vahhabi A, Seyedzadeh SJ, et al. Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sci. 2020;249:117447.
Kawasaki R, Sasaki Y, Katagiri K, Mukai SA, Sawada S, Akiyoshi K. Magnetically guided protein transduction by hybrid nanogel chaperones with iron oxide nanoparticles. Angew Chem Int Ed. 2016;55:11377–81.
Ueda T, Lee SJ, Nakatani Y, Ourisson G, Sunamoto J. Coating of POPC giant liposomes with hydrophobized polysaccharide. Chem Lett. 1998;5:417–8.
Mizuta R, Sasaki Y, Kawasaki R, Katagiri K, Sawada S, Mukai SA, et al. Magnetically navigated intracellular delivery of extracellular vesicles using amphiphilic nanogels. Bioconj Chem. 2019;30:2150–5.
Sancho-Albero M, Encabo-Berzosa MDM, Beltrán-Visiedo M, Fernández-Messina L, Sebastián V, Sánchez-Madrid F, et al. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale. 2019;11:18825–36.
Cao Y, Wu T, Zhang K, Meng X, Dai W, Wang D, et al. Engineered exosome-mediated near-infrared-II region V2C quantum dot delivery for nucleus-target low-temperature photothermal therapy. ACS Nano. 2019;13:1499–510.
Ando M, Akiyama M, Okuno D, Hirano M, Ide T, Sawada S, et al. Liposome chaperon in cell-free membrane protein synthesis: one-step preparation of KcsA-integrated liposomes and electrophysiological analysis by the planar bilayer method. Biomater Sci. 2015;4:258–64.
Lasitza‐Male T, Bartels K, Jungwirth J, Wiggers F, Rosenblum G, Hofmann H, Löw C. Membrane chemistry tunes the structure of a peptide transporter. Angew Chem Int Ed Eng. 2020;591:19121–28.
Niwa T, Sasaki Y, Uemura E, Nakamura S, Akiyama M, Ando M, et al. Comprehensive study of liposome-assisted synthesis of membrane proteins using a reconstituted cell-free translation system. Sci Rep. 2015;5:18025.
Jacobs M, Boyd MA, Kamat NP. Diblock copolymers enhance folding of a mechanosensitive membrane protein during cell-free expression. Proc Natl Acad Sci. 2019;116:4031–6.
Nallani M, Andreasson-Ochsner M, Tan CWD, Sinner E, Wisantoso Y, Geifman-Shochat S, et al. Proteopolymersomes: in vitro production of a membrane protein in polymersome membranes. Biointerphases. 2011;6:153–7.
Isaksson S, Watkins EB, Browning KL, Lind TK, Cárdenas M, Hedfalk K, et al. Protein-containing lipid bilayers intercalated with size-matched mesoporous silica thin films. Nano Lett. 2017;17:746–85.
Hurtig J, Chiu DT, Onfelt B. Intercellular nanotubes: insights from imaging studies and beyond. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:260–76.
Jorsh IK, Daste F, Gallop JL. Membrane curvature in cell biology: an integration of molecular mechanisms. J Cell Biol. 2016;214:375–87.
Nomura F, Honda M, Takeda S, Inaba T, Takiguchi K, Itoh TJ, et al. Morphological and topological transformation of membrane vesicles. J Biol Phys. 2002;28:225–35.
Dimova R, Lipowsky R. Giant vesicles exposed to aqueous two-phase systems: membrane wetting, budding processes, and spontaneous tubulation. Adv Mater Interfaces. 2017;4:16600451.
Karlsson A, Karlsson R, Karlsson M, Cans AS, Strömberg A, Ryttsén F, et al. Networks of nanotubes and containers. Nature. 2001;490:150–2.
Gerbrand K, Martijn V, Bas H, Marileen D. Membrane tube formation from giant vesicles by dynamic association of motor proteins. Proc Natl Acad Sci. 2003;100:15583–8.
Hayashi M, Nishiyama M, Kazayama Y, Toyota T, Harada Y, Takiguchi K. Reversible morphological control of tubulin-encapsulating giant liposomes by hydrostatic pressure. Langmuir. 2016;32:3794–802.
Brazhnik KP, Vreeland WN, Hutchison JB, Kishore R, Wells J, Helmerson K, et al. Directed growth of pure phosphatidylcholine nanotubes in microfluidic channels. Langmuir. 2005;21:10814–7.
Fu M, Li Q, Sun B, Yang Y, Dai L, Nylander T, et al. Disassembly of dipeptide single crystals can transform the lipid membrane into a network. ACS Nano. 2017;11:7349–54.
Koksal ES, Liese S, Kantarci I, Olsson R, Carlson A, Gozen I. Nanotube-mediated path to protocell formation. ACS Nano. 2019;13:6867–78.
Castillo JA, Narciso DM, Hayes MA. Bionanotubule formation from surface-attached liposomes using electric field. Langmuir. 2009;25:391–6.
Sekine Y, Abe K, Shimizu Z, Sasaki Y, Sawada S, Akiyoshi K. Shear flow-induced nanotubulation of surface-immobilized liposomes. RSC Adv. 2012;2:2682–4.
Sasaki Y, Akiyoshi K. PCT Int. Appl. JP 2011;06933:PCT/JP2011/069337.
Schulz M, Olubummo A, Binder WH. Beyond the lipid-bilayer: interaction of polymers and nanoparticles with membranes. Soft Matter. 2012;8:4849–64.
Stachowiak JC, Hayden CC, Sasaki DY. Steric confinement of proteins on lipid membranes can drive curvature and tubulation. Proc Natl Acad Sci. 2010;107:7781–6.
Safinya CR, Raviv U, Needleman DJ, Zidovska A, Choi MC, Ojeda-Lopez MA, et al. Nanoscale assembly in biological systems: from neuronal cytoskeletal proteins to curvature stabilizing lipids. Adv Mater. 2011;23:2260–70.
Zuraw-Weston S, Wood DA, Torres IK, Li YW, Wang LS, Jiang Z, et al. Nanoparticles binding to lipid membranes: from vesicle-based gels to vesicle tubulation and destruction. Nanoscale. 2019;11:18464–74.
Shimada N, Kinoshita H, Umegae T, Azumai S, Kume N, Ochiai T, et al. Cationic copolymer-chaperoned 2D–3D reversible conversion of lipid membranes. Adv Mater. 2019;31:1904032.
Doosti BA, Pezeshkian W, Btuhn DS, Ipsen JH, Khandelia H, Jeffries GD, et al. Membrane tubulation in lipid vesicles triggered by the local application of calcium ions. Langmuir. 2017;33:11010–7.
Acknowledgements
This work was financially supported by a Grant-in Aid from the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP16H03842 (YS). We thank KA for kind support and Dr. Shinichi Sawada and Dr. Sada-atsu Mukai for their contributions. We thank Michael Scott Long from the Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sasaki, Y., Akiyoshi, K. Design and function of smart biomembrane nanohybrids for biomedical applications: review. Polym J 53, 587–592 (2021). https://doi.org/10.1038/s41428-020-00453-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-00453-z