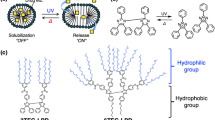
Abstract
This review summarizes the development of pH-responsive polymers and their main applications. Random pH-responsive copolymers have been prepared via conventional free radical polymerization from a pH-responsive pendant fatty acid-containing monomer (AaU) and a permanent water-soluble pendant sulfonate-containing monomer (AMPS). In water, the resulting copolymers, P(A/AaU), form single polymer chain (unimer) micelles under acidic conditions due to intrapolymer hydrophobic interactions between the protonated AaU units. The surfaces of the unimer micelles are covered with hydrophilic AMPS units, which provide colloidal stabilization. Under basic conditions, the P(A/AaU) polymer chains expand as a result of the electrostatic repulsive interactions between the pendant ionized fatty acids and the sulfonate anions. Through the use of a pH-responsive AaU homopolymer, pH-responsive sunscreen was developed. Although pH-responsive sunscreen shows waterproof properties under neutral conditions, it disperses under weakly basic conditions such as soap water. Therefore, pH-responsive sunscreen resists sweat but can be washed off using soap water. pH-responsive diblock copolymers composed of a PAMPS block and a pH-responsive pendant fatty acid-containing block were prepared via controlled radical polymerization. In water, the block copolymers form interpolymer micelles under acidic conditions due to hydrophobic interactions between the protonated pendant fatty acid groups, whereas the polymer micelles dissociate under basic conditions. Finally, this review also discusses pH-responsive gelling agents based on ABA triblock copolymers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Strauss UP, Jackson EG, Polysoaps I. Viscosity and solubilization studies on an n-dodecyl bromide addition compound of poly-2-vinylpyridine. J Polym Sci. 1951;6:649–59.
Jackson EG, Strauss UP. Polysoaps. II. Effect of added hydrocarbon on reduced viscosity of an n-dodecyl bromide addition compound of poly-2-vinylpyridine. J Polym Sci. 1951;7:473–84.
Morishima Y, Tanaka T, Itoh Y, Nozakura S. Amphiphilic copolymers consisting of vinylphenanthrene and cationic segments. Fluorescence quenching amphiphilic quenchers aqueous media. Poly J. 1982;14:861–8.
Morishima, Y In Functional Monomers and Polymers, 2nd ed.; Takemoto, K, Ottenbrite, RM, Kamachi, M, Eds.; Marcel Dekker: New York, 1997; 455.
Terashima T. Functional spaces in star and single-chain polymers via living radical polymerization. Poly J 2014;46:664–73.
Yusa S, Konishi Y, Mitsukami Y, Yamamoto T, Morishima Y. pH-responsive micellization of amine-containing cationic diblock copolymers prepared by reversible addition-fragmentation chain transfer (RAFT) radical polymerization. Polym J. 2005;37:480–8.
Yusa S, Yamago S, Sugahara M, Morikawa S, Yamamoto T, Morishima Y. Thermo-responsive diblock copolymers of poly(N-isopropylacrylamide) and poly(N-vinyl-2-pyrroridone) synthesized via organotellurium-mediated controlled radical polymerization (TERP). Macromolecules 2007;40:5907–15.
Guragain S, Bastakoti BP, Ito M, Yusa S, Nakashima K. Aqueous polymeric micelles of poly[N-isopropylacrylamide-b-sodium 2-(acrylamido)-2-methylpropanesulfonate] with a spiropyran dimer pendant: quadruple stimuli-responsiveness. Soft Matter. 2012;8:9628–34.
Yusa S, Sakakibara A, Yamamoto T, Morishima Y. Reversible pH-induced formation and disruption of unimolecular micelles of an amphiphilic polyelectrolyte. Macromolecules 2002;35:5243–9.
Ohno S, Ishihara K, Yusa S. Formation of polyion complex (PIC) micelles and vesicles with anionic pH-responsive unimer micelles and cationic diblock copolymers in water. Langmuir 2016;32:3945–53.
Veregin RPN, Odell PG, Michalak LM, Georges MK. Molecular weight distributions in nitroxide-mediated living free radical polymerization: kinetics of the slow equilibria between growing and dormant chains. Macromolecules 1996;29:3346–52.
Wang JS, Matyjaszewski K. “Living”/controlled radical polymerization. Transition-metal-catalyzed atom transfer radical polymerization in the presence of a conventional radical initiator. Macromolecules 1995;28:7572–3.
Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: the RAFT process. Macromolecules 1998;31:5559–62.
Yamago S, Iida K, Yoshida J. Tailored synthesis of structurally defined polymers by organotellurium-mediated living radical polymerization (TERP): synthesis of poly(meth)acrylate derivatives and their di- and triblock copolymers. J Am Chem Soc. 2002;124:13666–7.
Yusa S, Shimada Y, Mitsukami Y, Yamamoto T, Morishima Y. pH-responsive micellization of amphiphilic diblock copolymers synthesized via reversible addition-fragmentation chain transfer polymerization. Macromolecules 2003;36:4208–15.
Aota H, Araki S, Morishima Y, Kamachi M. Photoinduced electron transfer to methylviologen from zinc(II) tetraphenylporphyrin compartmentalized in unimer micelles of amphiphilic polyelectrolytes. Macromolecules 1997;30:4090–6.
Tominaga Y, Mizuse M, Hashidzume A, Morishima Y, Sato T. Flower micelle of amphiphilic random copolymers in aqueous media. J Phys Chem B. 2010;114:11403–8.
Hashidzume A, Mizusaki M, Yoda K, Morishima Y. Interaction of unimolecular micelles of hydrophobically modified polyelectrolytes with nonionic/ionic mixed surfactant micelles. Langmuir 1999;15:4276–82.
Terashima T, Sugita T, Fukae K, Sawamoto M. Synthesis and single-chain folding of amphiphilic random copolymers in water. Macromolecules 2014;47:589–600.
Terashima T. Controlled self-assembly of amphiphilic random copolymers into folded micelles and nanostructure materials. J Oleo Sci. 2020;69:529–38.
Morishima Y. Unimolecular micelles of hydrophobically modified polysulfonates: potential utility for novel photochemical systems. Trends Polym Sci. 1994;2:31–6.
Morishima Y, Kamachi M. Spectroscopic studies of unimer micelles of hydrophobically modified polysulfonates. Polym Eng Sci. 1994;71:271–2.
Morishima Y, Nomura S, Ikeda T, Seki M, Kamachi M. Characterization of unimolecular micelles of random copolymers of sodium 2-(Acrylamido)-2-methylpropanesulfonate and methacrylamides bearing bulky hydrophobic substituents. Macromolecules 1995;28:2874–81.
Morishima Y, Aota H, Saegusa K, Kamachi M. Long-lived porphyrin cation radicals protected in unimer micelles of hydrophobically-modified polyelectrolytes. Macromolecules 1996;29:6505–9.
Yamamoto H, Morishima Y. Effect of hydrophobe content on Intra- and Interpolymer self-associations of hydrophobically modified poly(sodium 2-(acrylamido)-2-methylpropanesulfonate) in water. Macromolecules 1999;32:7469–75.
Kaditi E, Mountrichas G, Pispas S, Demetzos C. Block copolymers for drug delivery nano systems (DDnSs). Curr Med Chem. 2012;19:5088–100.
Yusa S, Sakakibara A, Yamamoto T, Morishima Y. Fluorescence studies of pH-responsive unimolecular micelles formed from smphiphilic polysulfonates possessing long-chain alkyl carboxyl pendants. Macromolecules 2002;35:10182–8.
Yusa S. Formation and disruption of unimolecular micelle triggered by variation of pH. Kobunshi Ronbunshu. 2004;61:399–407.
de Gruijl FR. Skin cancer and solar UV radiation. Eur J Cancer Care. 1999;35:2003–9.
Moan J, Dahlback A, Setlow RB. Epidemiological support for an hypothesis for melanoma induction indicating a role for UVA radiation. Photochem Photobio. 1999;70:243–7.
Kerr RA. Antarctic ozone hole is still deepening. Science 1986;232:1602.
Sumida Y, Yamashita H, Shimizu K, Takatsu A. Development of the cosmetics formulation with the aim of adding functionality. some examples of development on skin-care cosmetics. Found Make- Cosmetics Fragr J 2004;32:33–9.
Su W, Zhang J, Feng Z, Chen T, Ying P, Li C. Surface phases of TiO2 nanoparticles studied by UV raman spectroscopy and FT-IR spectroscopy. J Phys Chem C. 2008;112:7710–6.
Osawa T, Sogabe A, Shirao M, Nishihama S, Kaneda I, Yusa S. Development of a water-resistant and detergent-washable powder coated with a stimuli-responsive polymer and its application to suncare products. IFSCC Mag. 2009;12:3–7.
Osawa T. Development of pH-responsive material and the future prospect. Fragr J 2010;38:26–30.
Usui, Y; Yusa, S ABA Triblock Copolymer, Thickener, and Aqueous Composition. Japan, JP2013241567 A 2013-12-5.
Taktak FF, Buetuen V. Synthesis and physical gels of pH- and thermo-responsive tertiary amine methacrylate based ABA triblock copolymers and drug release studies. Polymer 2010;51:3618–26.
Zhao X, Liu W, Chen D, Lin X, Lu WW. Effect of block order of ABA- and BAB-type NIPAAm/HEMA triblock copolymers on thermoresponsive behavior of solutions. Macromol Chem Phys. 2007;208:1773–81.
Acknowledgements
This research was partially supported by KAKENHI grants (21H02005, 21K19931, 21H05027) from the Japan Society for the Promotion of Science (JSPS), JSPS Bilateral Joint Research Projects (JPJSBP120203509), and a project (JPNP20003) commissioned by the New Energy and Industrial Technology Development Organization (NEDO). and the Cooperative Research Program of “Network Joint Research Center for Materials and Devices (20214044).” The author would like to thank Enago for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yusa, Si. Development and application of pH-responsive polymers. Polym J 54, 235–242 (2022). https://doi.org/10.1038/s41428-021-00576-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-021-00576-x