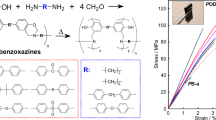
Abstract
Performing investigations on the structure-property relationship of poly(silane arylether arylacetylene)s (PSEAs) is very important and instructive for designing novel high-performance polymers. In this work, three bifunctional acetylene-terminated triphenyl-ether monomers with either para-acetylene, meta-acetylene or ortho-acetylene, i.e., 1,3-bis(4'-ethynylphenoxy)benzene (pmp-BEPB), 1,3-bis(3'-ethynylphenoxy)benzene (mmm-BEPB) and 1,3-bis(2'-ethynylphenoxy)benzene (omo-BEPB) were synthesized and used to prepare three PSEA resins, namely, pmp-PSEA, mmm-PSEA and omo-PSEA. The PSEA resins were characterized by 1H-nuclear magnetic resonance spectroscopy, Fourier transform infrared spectroscopy and X-ray diffraction analysis, and the properties were explored. The resins possess a wide processing window in the following order: pmp-PSEA < mmm-PSEA < omo-PSEA. The DSC results revealed that pmp-PSEA shows the highest reactivity, followed by omo-PSEA and mmm-PSEA. In the cured networks of the resins, the densities increase in the following order: pmp-PSEA-C < mmm-PSEA-C < omo-PSEA-C. Compared with pmp-PSEA-C, mmm-PSEA-C possesses better mechanical properties with a flexural strength of 66.5 MPa and a flexural modulus of 3.71 GPa. The decomposition temperatures of 5% weight loss (Td5) of pmp-PSEA-C and mmm-PSEA-C are over 530 °C.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Itoh M, Iwata K, Ishikawa J, Sukawa H, Kimura H, Okita K. Various silicon-containing polymers with Si(H)-C≡C units. J Polym Sci Polym Chem. 2001;39:2658–69.
Gao M, Shang C, Li J, Han G, Tang J, Yuan Q, et al. Synthesis and characterization of block copolymers of poly(silylene diethynylbenzene) and poly(silylene dipropargyl aryl ether). Polymers. 2021;13:1511.
Chen H, Xin H, Lu J, Tang J, Yuan Q, Huang F. Synthesis and properties of poly(dimethylsilylene-ethynylene-phenoxyphenoxyphenylene-ethynylene). High Perform Polym. 2017;29:595–601.
Ma M, Li C, Liu X, Yuan Q, Huang F. Synthesis and properties of a silicon-containing arylacetylene resin with 2,6-diphenoxypyridine unit. ChemistrySelect. 2020;5:1146–52.
Ma M, Gong C, Li C, Yuan Q, Huang F. The synthesis and properties of silicon-containing arylacetylene resins with rigid-rod 2,5-diphenyl-[1,3,4]-oxadiazole moieties. Eur Polym J. 2021;143:110192.
Ma M, Liu X, Li C, Qiao Z, Yuan Q, Huang F. Effects of pendant side groups on the properties of the silicon-containing arylacetylene resins with 2,5-diphenyl-[1,3,4]-oxadiazole moieties. RSC Adv. 2021;11:19656–65.
Ma M, Liu X, Li C, Yuan Q, Huang F. A high-performance silicon-containing arylacetylene resin with conjugated naphthalene rings. High Perform Polym. 2022;34:24–32.
Li C, Han G, Ma M, Tang J, Huang F. Synthesis and properties of poly(silylenebis(ethynylphenoxy)diphenylsulfone. Polym Bull. 2023;80:349–63.
Gong C, Huang X, Li J, Lv S, Zhou Y, Tang J, et al. Perfluorocyclobutyl aryl ether-based poly(silylene arylacetylene)s with a low dielectric constant for advanced wave-transparent composites. Eur Polym J. 2022;181:111655.
Sidra LR, Chen G, Mushtaq N, Ma K, Bashir B, Fang X. Processable poly(benzoxazole imide)s derived from asymmetric benzoxazole diamines containing 4-phenoxy aniline: synthesis, properties and the isomeric effect. Polym Chem. 2018;9:2785–96.
Sidra LR, Chen G, Li C, Mushtaq N, Ma K, Fang X. Processable, high Tg polyimides from unsymmetrical diamines containing 4-phenoxy aniline and benzimidazole moieties. Polymer. 2018;148:228–38.
Jiao Y, Chen G, Mushtaq N, Zhou H, Chen X, Li Y, et al. Synthesis and properties of poly(benzoxazole imide)s derived from two isomeric diamines containing a benzoxazole moiety. Polym Chem. 2020;11:1937–46.
Liu Y, Xie F, Huang J, Tan J, Chen C, Jiang L, et al. The effect of molecular isomerism on the barrier properties of polyimides: perspectives from experiments and simulations. Polymers. 2021;13:1749.
Baqar M, Agag T, Huang R, Maia J, Qutubuddin S, Ishida H. Mechanistic pathways for the polymerization of methylol-functional benzoxazine monomers. Macromolecules. 2012;45:8119–25.
Zhang K, Froimowicz P, Han L, Ishida H. Hydrogen-bonding characteristics and unique ring-opening polymerization behavior of ortho-methylol functional benzoxazine. J Polym Sci Polym Chem. 2016;54:3635–42.
Kolanadiyil SN, Minami M, Endo T. Synthesis and thermal properties of difunctional benzoxazines with attached oxazine ring at the para-, meta-, and ortho-position. Macromolecules. 2017;50:3476–88.
Osaka I, Abe T, Shinamura S, Takimiya K. Impact of isomeric structures on transistor performances in naphthodithiophene semiconducting polymers. J Am Chem Soc. 2011;133:6852–60.
Más-Montoya M, Ortiz RP, Curiel D, Espinosa A, Allain M, Facchetti A, et al. Isomeric carbazolocarbazoles: synthesis, characterization and comparative study in organic field effect transistors. J Mater Chem C. 2013;1:1959–69.
Zhao Z, Zhang F, Zhang X, Yang X, Li H, Gao X, et al. 1,2,5,6-Naphthalenediimide based donor-acceptor copolymers designed from isomer chemistry for organic semiconducting materials. Macromolecules. 2013;46:7705–14.
Osaka I, Houchin Y, Yamashita M, Kakara T, Takemura N, Koganezawa T, et al. Contrasting effect of alkylation on the ordering structure in isomeric naphthodithiophene-based polymers. Macromolecules. 2014;47:3502–10.
Singh R, Pagona G, Gregoriou VG, Tagmatarchis N, Toliopoulos D, Han Y, et al. The impact of thienothiophene isomeric structures on the optoelectronic properties and photovoltaic performance in quinoxaline based donor-acceptor copolymers. Polym Chem. 2015;6:3098–109.
Wang J, Zhang J, Xiao Y, Xiao T, Zhu R, Yan C, et al. Effect of isomerization on high-performance nonfullerene electron acceptors. J Am Chem Soc. 2018;140:9140–7.
Zheng R, Guo Q, Hao D, Zhang C, Xue W, Huang H, et al. Naphthalene core-based noncovalently fused-ring electron acceptors: effects of linkage positions on photovoltaic performances. J Mater Chem C. 2019;7:15141–7.
Wang H, Wang C, Chen Y, Cao J, Ren X, Hong W, et al. Synthesis and molecular properties of isomeric thienoisoindigo. J Mater Chem C. 2021;9:13218–25.
Shi Y, Li W, Wang X, Tu L, Li M, Zhao Y, et al. Isomeric acceptor-acceptor polymers: enabling electron transport with strikingly different semiconducting properties in n‑channel organic thin-film transistors. Chem Mater. 2022;34:1403–13.
Zhang J, Huang J, Du W, Huang F, Du L. Thermal stability of the copolymers of silicon-containing arylacetylene resin and acetylene-functional benzoxazine. Polym Degrad Stabil. 2011;96:2276–83.
Shen Y, Yuan Q, Huang F, Du L. Effect of neutral nickel catalyst on cure process of silicon-containing polyarylacetylene. Thermochim Acta. 2014;590:66–72.
Itoh M, Inoue K, Iwata K, Mitsuzuka M, Kakigano T. New highly heat-resistant polymers containing silicon: poly(silyleneethynylenephenyleneethynylene)s. Macromolecules. 1997;30:694–701.
Li J, Lv S, Gong C, Zhou Y, Huang F. Effect of the linking positions on the benzene ring on properties of poly(silane arylether arylacetylene)s. J Polym Sci. 2022;60:3232–43.
Okazakia T, Nakagawaa M, Futemma T, Kitagawa T. NMR and DFT studies on persistent carbocations derived from benzo[kl]xanthene, dibenzo[d,d’]benzo[1,2-b:4,3-b’]difuran, and dibenzo[d,d’]benzo[1,2-b:4,5-b’]difuran in superacidic media. J Phys Org Chem. 2016;29:107–11.
Wie JJ, Wang DH, Lee KM, Tan L, White TJ. Molecular engineering of azobenzene-functionalized polyimides to enhance both photomechanical work and motion. Chem Mater. 2014;26:5223–30.
Sharmoukh W, Ko KC, Noh C, Lee JY, Son SU. Designed synthesis of multi-electrochromic systems bearing diaryl ketone and isophthalates. J Org Chem. 2010;75:6708–11.
Yang R, Ding L, Chen W, Chen L, Zhang X, Li J. Chain folding in main-chain liquid crystalline polyester with strong π-π interaction: an efficient β‑nucleating agent for isotactic polypropylene. Macromolecules. 2017;50:1610–7.
Ni Y, Li Q, Chen L, Wu W, Qin Z, Zhang Y, et al. Semi-aromatic copolyesters with high strength and fire safety via hydrogen bonds and π-π stacking. Chem Eng J. 2019;374:694–705.
Pichon PG, David L, Mechin F, Sautereau H. Morphologies of cross-linked segmented polyurethanes: evolution during maturation and consequences on elastic properties and thermal compressive fatigue. Macromolecules. 2010;43:1888–900.
Janicek M, Cermak R, Obadal M, Piel C, Ponizil P. Ethylene copolymers with crystallizable side chains. Macromolecules. 2011;44:6759–66.
Han G, Hou J, Wan L, Hao X, Liu X, Lv S, et al. Enhance high‑temperature mechanical performance of a silicon‑containing arylether arylacetylene resin with the aid of a terminal alkyne compound. J Polym Res. 2021;28:421.
Varley RJ, Dao B, Tucker S, Christensen S, Wiggins J, Dingemans T, et al. Effect of aromatic substitution on the kinetics and properties of epoxy cured tri-phenylether amines. J Appl Polym Sci. 2019;136:47383.
Li J, Gong C, Lv S, Huang F. Effects of side alkoxy groups on the arylacetylene unit on properties of poly(silylene arylacetylene)s. Eur Polym J. 2021;160:110774.
Luo J, Liu X, Ma M, Tang J, Huang F. Dendritic poly(silylene arylacetylene) resins based on 1,3,5-triethynylbenzene. Eur Polym J. 2020;129:109628.
Ramsdale-Capper R, Foreman JP. Internal antiplasticisation in highly crosslinked amine cured multifunctional epoxy resins. Polymer. 2018;146:321–30.
Kolanadiyil SN, Minami M, Endo T. Implementation of meta-positioning in tetrafunctional benzoxazines: synthesis, properties, and differences in the polymerized structure. Macromolecules. 2020;53:6866–86.
Martos A, Soto M, Schäfer H, Koschek K, Marquet J, Sebastián RM. Highly crosslinked polybenzoxazines from monobenzoxazines: the effect of meta-substitution in the phenol ring. Polymers. 2020;12:254.
Reyes LQ, Zhang J, Dao B, Varley RJ. Synthesis of tri-aryl ether epoxy resin isomers and their cure with diamino diphenyl sulphone. J Polym Sci. 2020;58:1410–25.
Acknowledgements
The authors gratefully acknowledge the support of the Fundamental Research Funds for the Central Universities (JKD01231701).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Gong, C., Tang, J. et al. Effect of substituted positions of acetylene groups in benzene rings on the properties of poly(silane arylether arylacetylene)s. Polym J 55, 1307–1315 (2023). https://doi.org/10.1038/s41428-023-00819-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00819-z